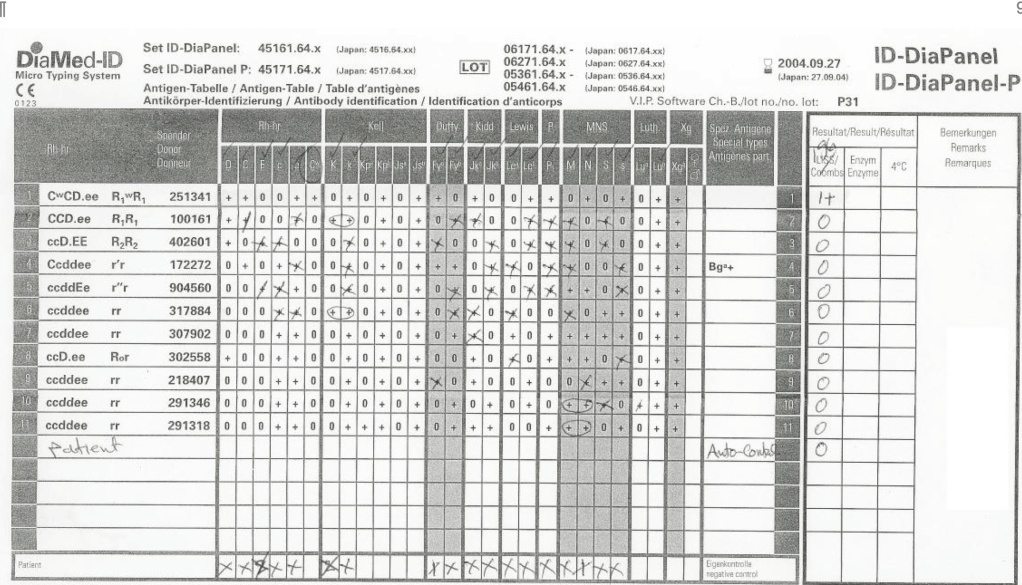
Rh immunoprophylaxis has big effect on the Transfusion Medicine operations. With a very active obstetrics program, there are numerous prenatal blood bank workups and administrations of Rh immune globulin RhIG—and consequently many antibody identifications. In fact at my previous position, the largest single source of antibody workups was from patients post-RhIG administration.
As a result of the antenatal RhIG use, there were numerous positive antibody screens. Our policy had been to always do a full antibody identification, both AHG and enzyme panels, and this made up the bulk of our antibody testing. In my experience, I have found other antibody specificities in some of these patients, including anti-Kell and anti-c, both of which could be very clinically significant.
As part of the workup, we also did ABO and extended Rh and Kell phenotyping, and thus identified rare Rh phenotypes (r’r’, -D-, etc.) and several Bombay phenotypes.
Unfortunately, I have been at institutions where they assumed a positive antibody screen in a patient with recent RhIG administration was passive anti-D and did NOT perform further testing.
If the patient needs blood and the antibody screen is positive, the antibody workup must be performed for routine release. This means about a 20-30 minutes delay in release until the antibody panels are completed and reviewed. Otherwise, blood can be released through an emergency protocol if the clinician accepts responsibility for incomplete testing at the time of release.
Many clinicians refused to do this emergency release and insisted on waiting for the antibody identification before accepting RBC components. They would not take the responsibility for the emergency release.
If we had done the antibody workup in these RhIG patients, we would have identified the anti-D, and I as the transfusion medicine physician would then advise the clinician to accept emergency release of RBCs while we complete the antibody workup.
For this reason alone, I insisted on a full antibody workup for all RhIG patients RhIG who exhibit a positive antibody screen. I would be comfortable in recommending emergency release if I had results of the previous antibody identification showing passive anti-D. The delay in release could adversely affect the patient’s outcome if there is active bleeding.
In summary, if a patient has a positive antibody screen post RhIG administration, you should still do ABID to rule out other antibodies and facilitate release of RBCs to a bleeding patient.