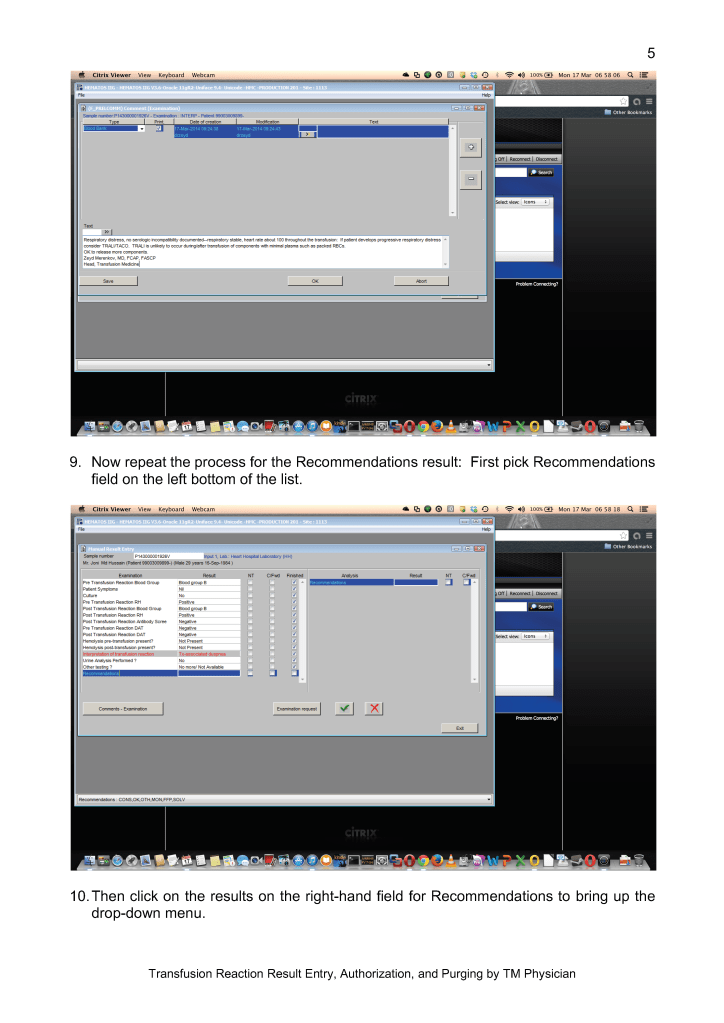
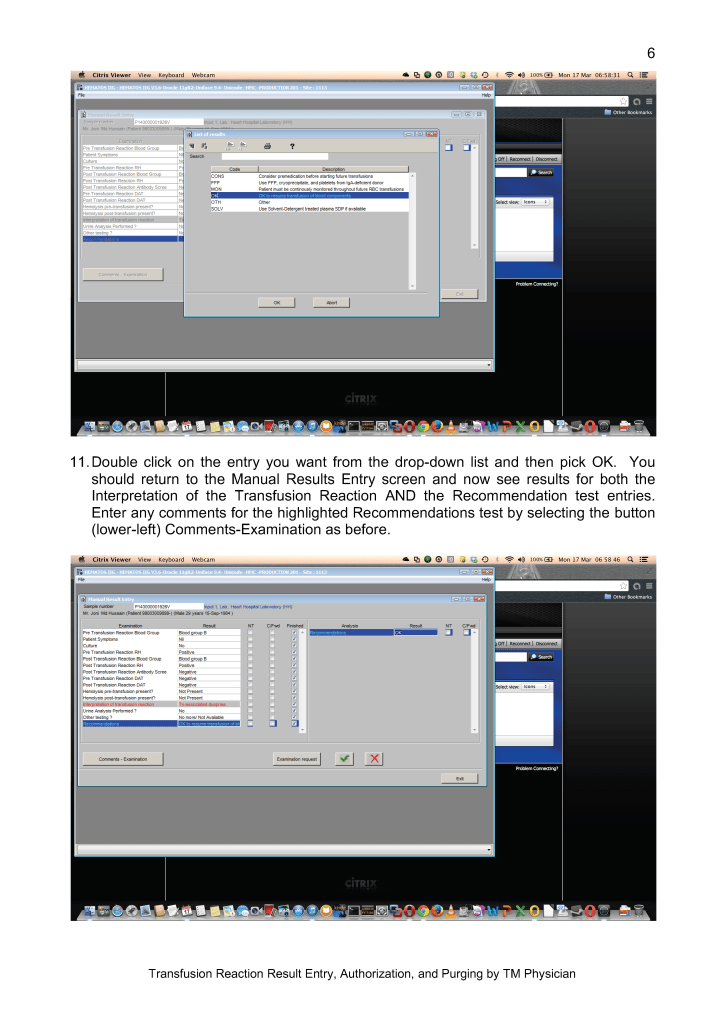
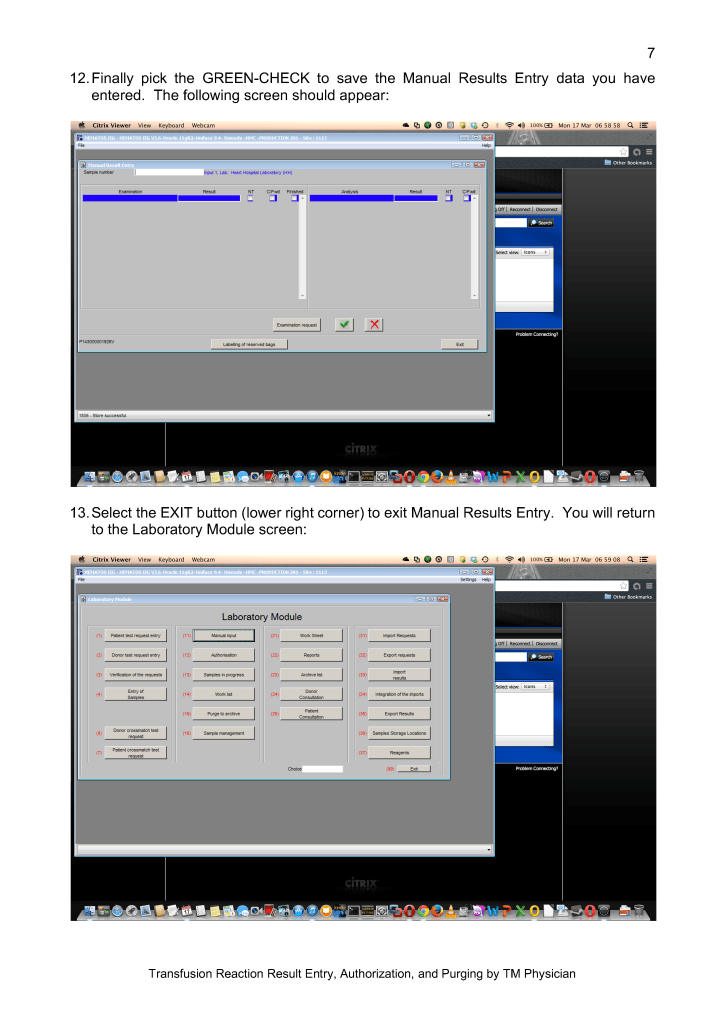
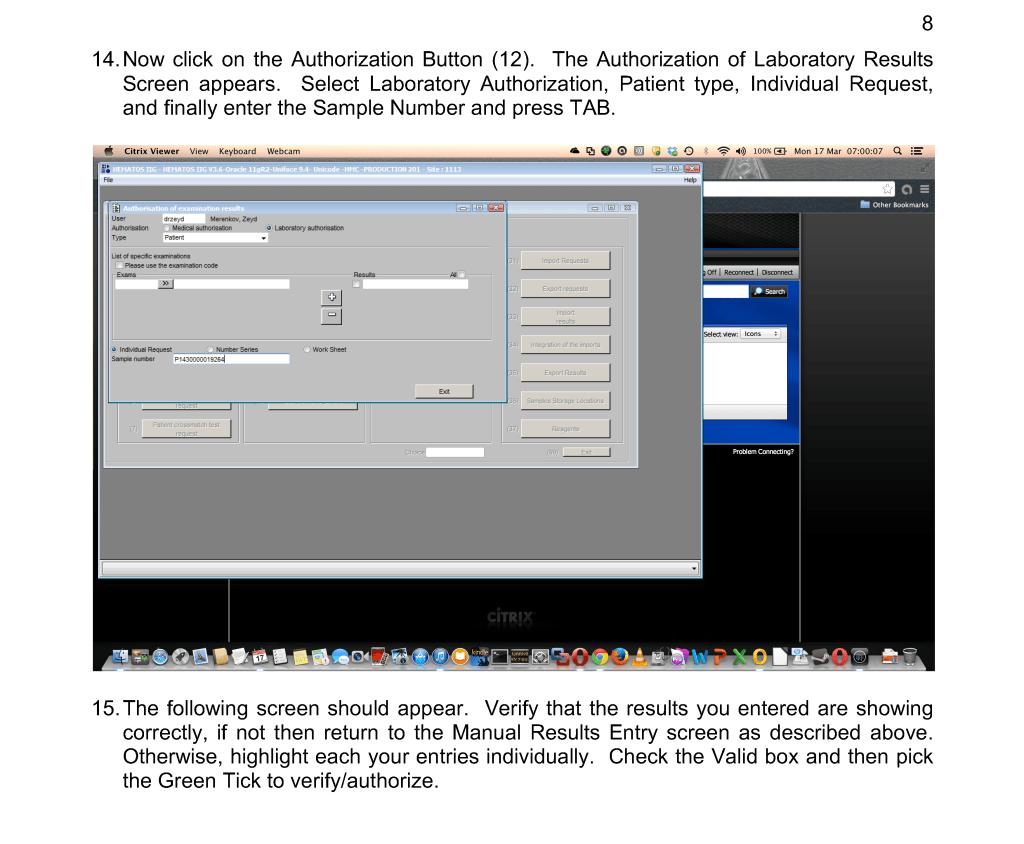
I originally adopted this technology because of the plethora of new emerging pathogens. In addition, I have been concerned about unknown pathogens that have not yet been discovered. It is not what we know, it is what we don’t know that bothered me.
It is now 10 years since I started using riboflavin-based pathogen inactivation. Our adoption of the technology was as follows:
- 2010 pooled buffy coat and apheresis platelets, both suspended in plasma
- 2012 whole-blood derived plasma and apheresis plasma
- 2015 pooled buffy coat and apheresis platelets, both suspended in platelet additive solution PAS
Buffy coat platelet pools and whole-blood-derived plasma were both prepared with automated blood component technology, originally with the Terumo Atreus and later with Terumo Reveos system. We were the first site worldwide to use automated production with the Mirasol system.
After 10 years and over 300,000 donor collections, no documented infectious agent transmission has been noted. Our average platelet loss has been 4%. There has been no increase in adverse reactions to plasma or platelets compared the time before we adopted these technologies. Physicians accepted the products readily.
Mirasol adoption allowed us to discontinue irradiation of platelets and extend our outdate to 7 days. We did not need a specific bacterial detection system. Pending regulations in the USA will require stringent bacterial detection processes that are not necessary if a pathogen-inactivation system is being used.
Terumo sent its own engineers to set up and validate the system. They also trained all the staff in the actual pathogen-inactivation processes and helped us to perform the validations.
When adopting pathogen inactivation, we compared Mirasol with its competitor and selected it for the following reasons:
- Loss of platelets is low (about 4%)—lower than its competitor product.
- There is no need to remove the riboflavin from the final product.
In our system, our goal was rapid processing of units. With Mirasol, we did not have remove the riboflavin from the final product. The competitor product requires at least 6 hours post-treatment to remove the psoralen agent. We could immediately use the Mirasol product after treatment!
In our Reveos-Mirasol system, we can process whole blood into packed red cells in SAGM, buffy coat platelets, and plasma in a total of 5 hours including all testing with Mirasol treatment and platelet additive solution PAS.
We originally used the system manually, but in 2013, Terumo in conjunction with Medinfo Hematos IIG developed an interface to the Mirasol illuminator. The latter device would transmit the successful completion of the illumination to the software. Any errors in the illumination would block release of the blood component from Medinfo. Medinfo also monitored the component volumes to prevent treatment of units outside Terumo’s recommendation ranges.
Adoption of platelet additive solution PAS gives us a final product with minimal residual plasma which potentially can reduce plasma reactions and TRALI/TACO. It also minimizes our need to reduce the volume of platelet components for pediatric patients, especially in cases with ABO-incompatible plasma
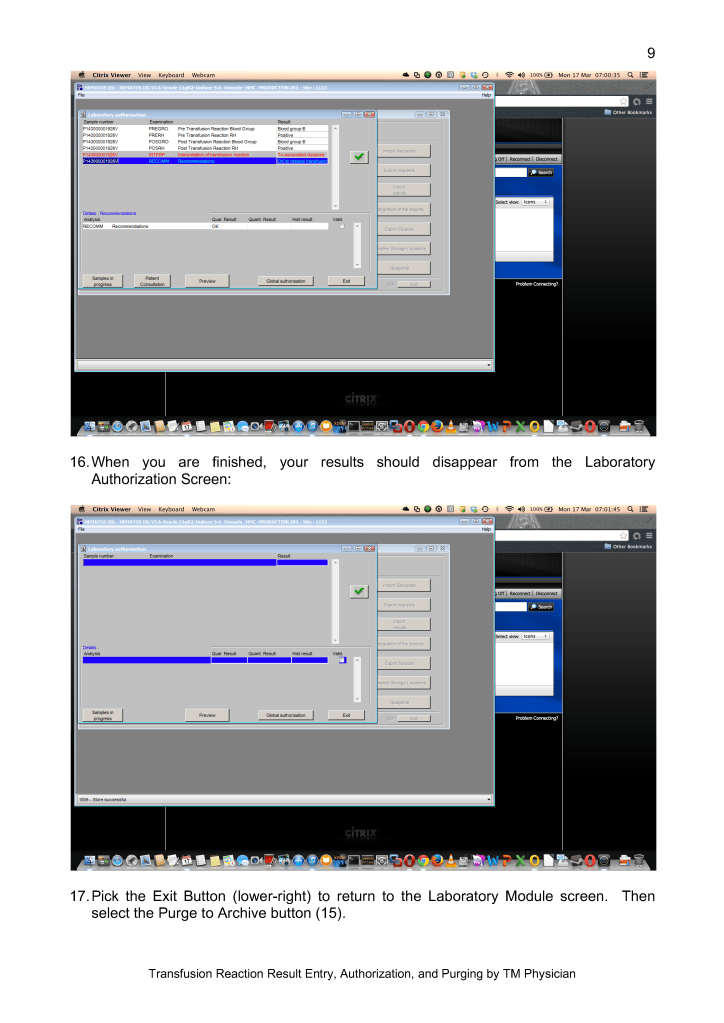
Adopting any system of pathogen inactivation requires meticulous monitoring of component volumes to ensure they are within the range for the treatment. The use of a blood bank software greatly facilitates this.
We make both buffy coat and apheresis platelets. The change from plasma-suspended to PAS-suspended platelets went smoothly. Special training for Trima apheresis staff to use the new processes was provided by Terumo.
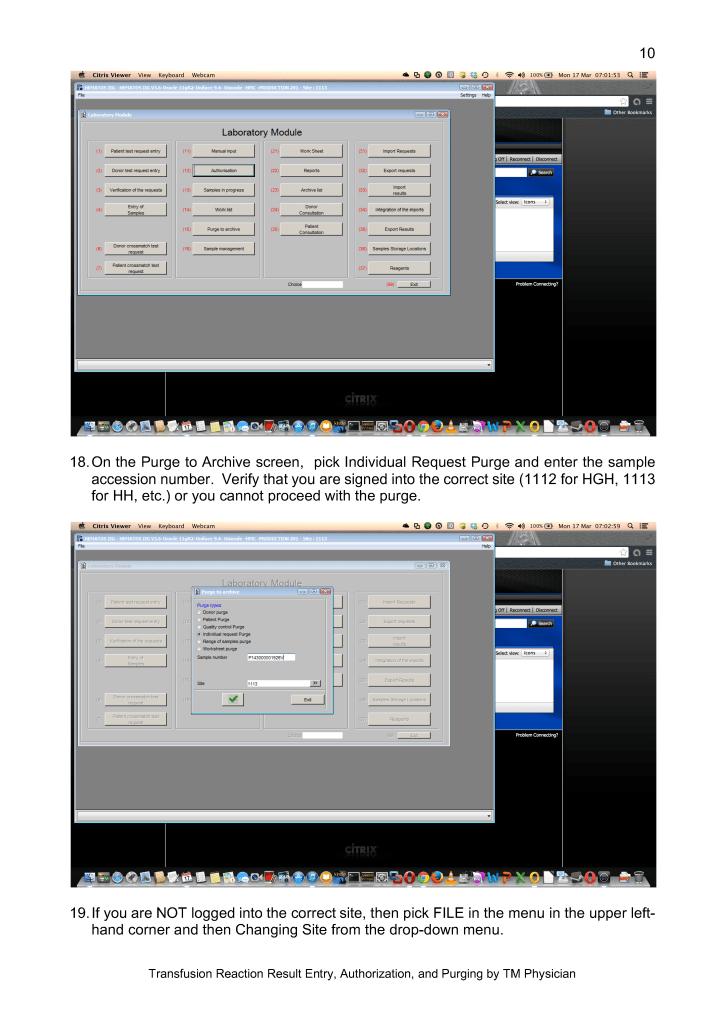
Throughout this time interval, Terumo has provided excellent technical support and educational activities for all staff. Despite the COVID pandemic, Terumo has been able to deliver supplies to meet our needs so there was no interruption in production.
We started COVID convalescent plasma CCP production at the end of winter 2020. We set up a parallel but separate quarantine system of collection and processing, originally manual but later controlled by the dedicated blood bank software Medinfo Hematos IIG. All CCP units have been treated with Mirasol.
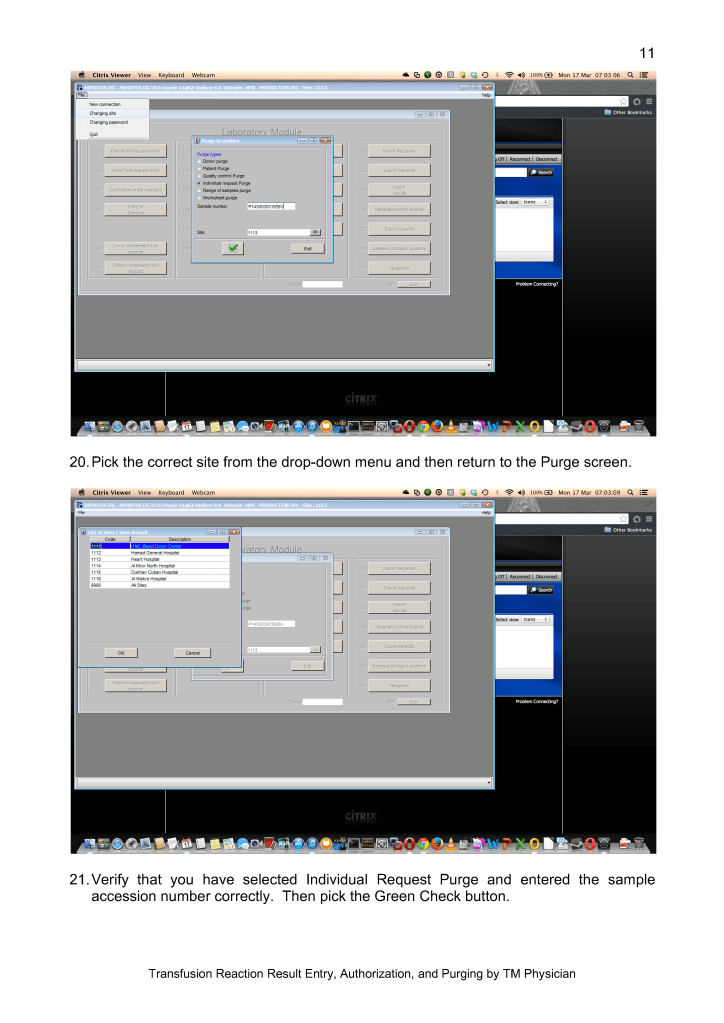
In the future, I hope Mirasol will close the loop by providing a pathogen-inactivation process for red blood cells so all components can be treated. The CE mark for pathogen inactivation of whole blood is exciting and I hope that component preparation from this product will be offered.
In summary, our blood bank system had an excellent, synergistic relationship with Terumo and Medinfo to provide the highest quality product that is currently licensed. I hope we will all continue to work together to improve the patient care.