This is a revised version of a previous post.



This is a revised version of a previous post.








This is an updated version of a previous post.

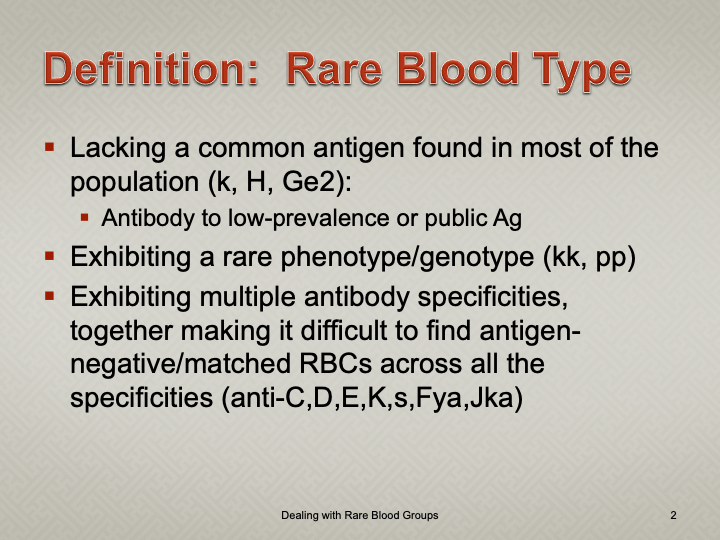
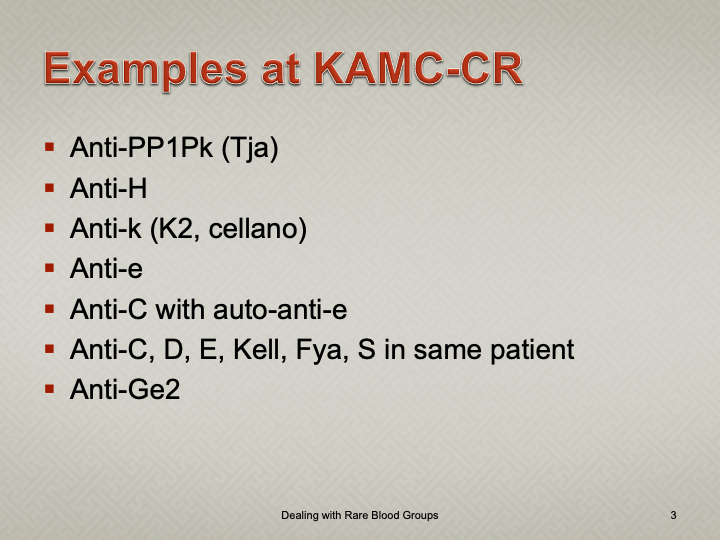
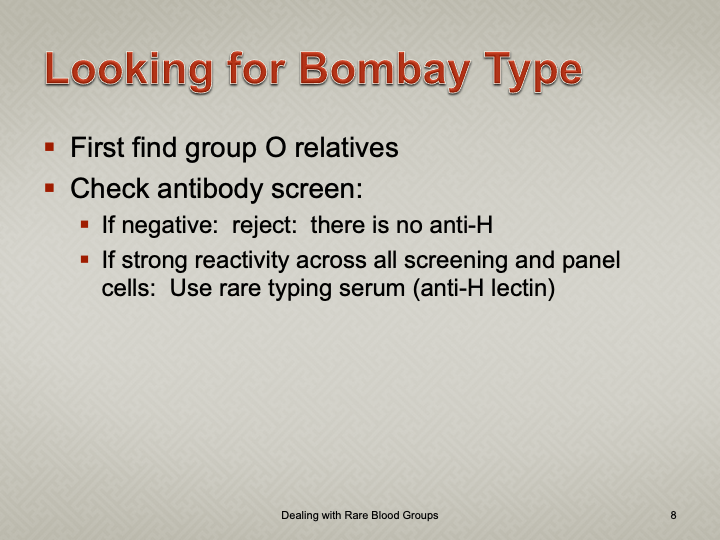
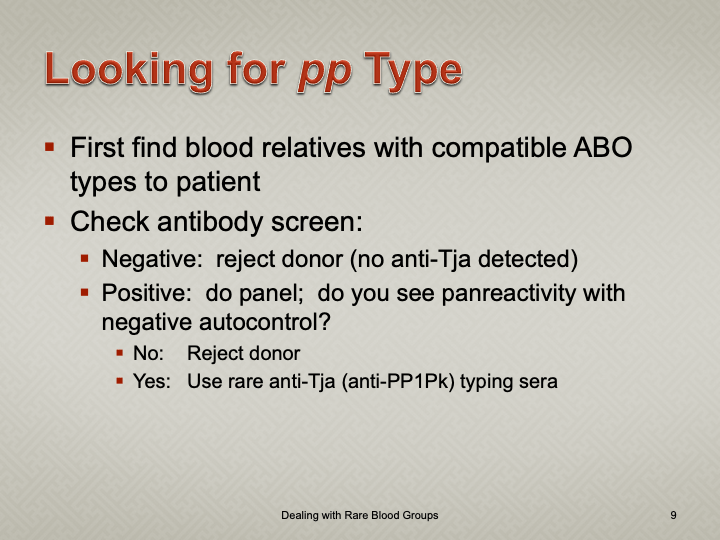


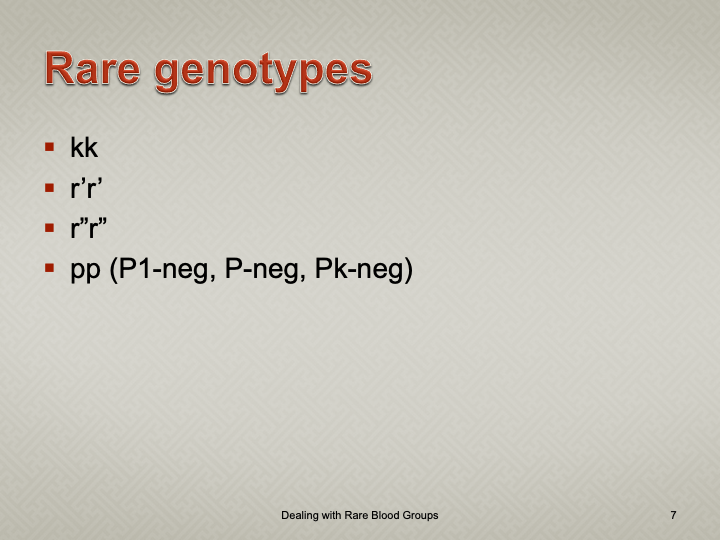
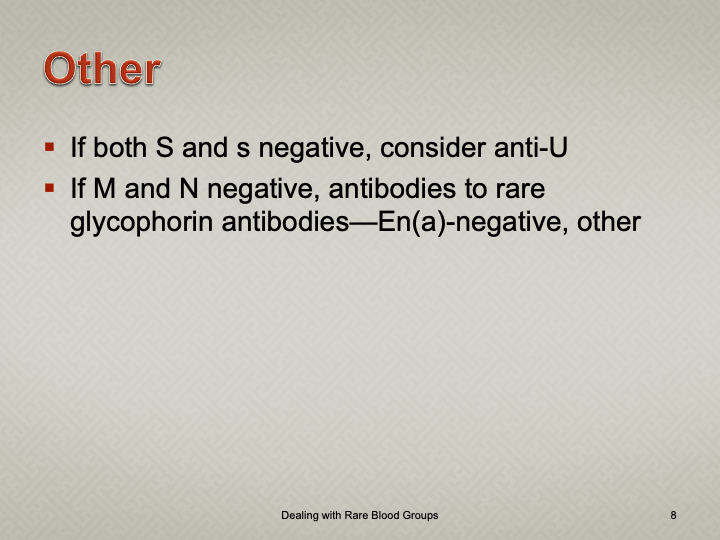
Enumeration: 5.8.2
Principle:
AABB requires that all donors be notified and counseled of abnormal test results in a timely manner. Notifications should be done based on required AABB and/or CE protocols including the requirements for follow-up testing at specified intervals.
Process:
References:
Enumeration: 5.8
Principle:
AABB requires that all donors be notified and counseled of abnormal test results in a timely manner. Notifications should be done based on required AABB and/or CE protocols including the requirements for follow-up testing at specified intervals.
Policy:
References:
5.7.1 PROCESS: Donor Marker Testing
Process:
References:
Enumeration: 5.7
Policy:
References:
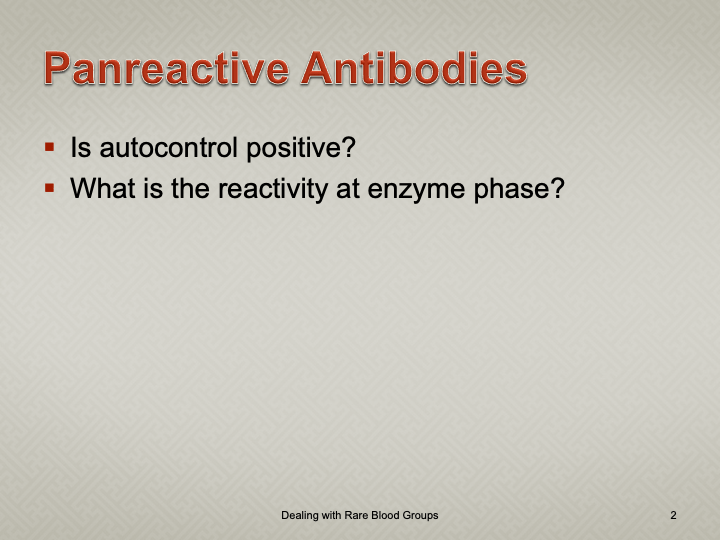
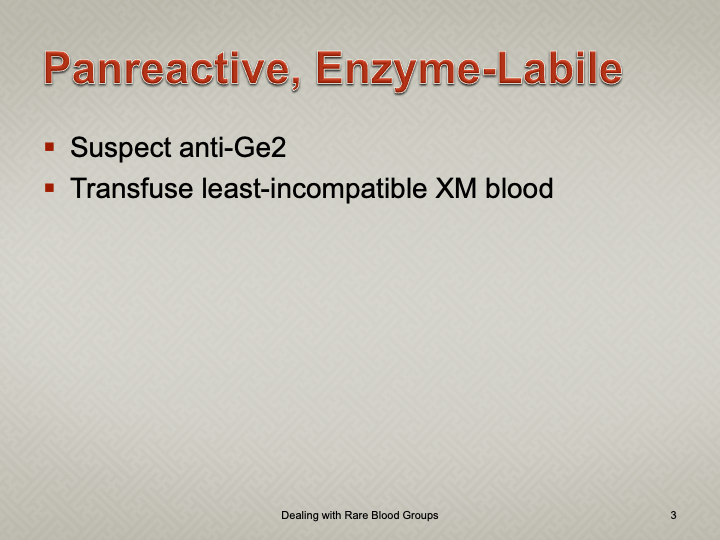
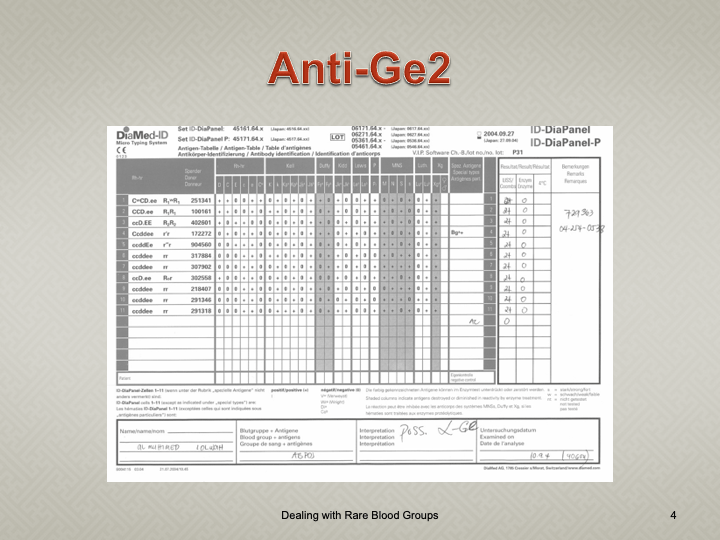
When I review nonspecific reactions on an antibody panel, I always check the outdate of the reagents and the age of the specimen. Outdating reagents may show weak reactivity. Old specimens may get contaminated and bacteria may alter the RBC surfaces (e.g. T-activation).
Here is an example of weakened reactions with an outdating antibody panel. This panel was used one-week before its expiration:
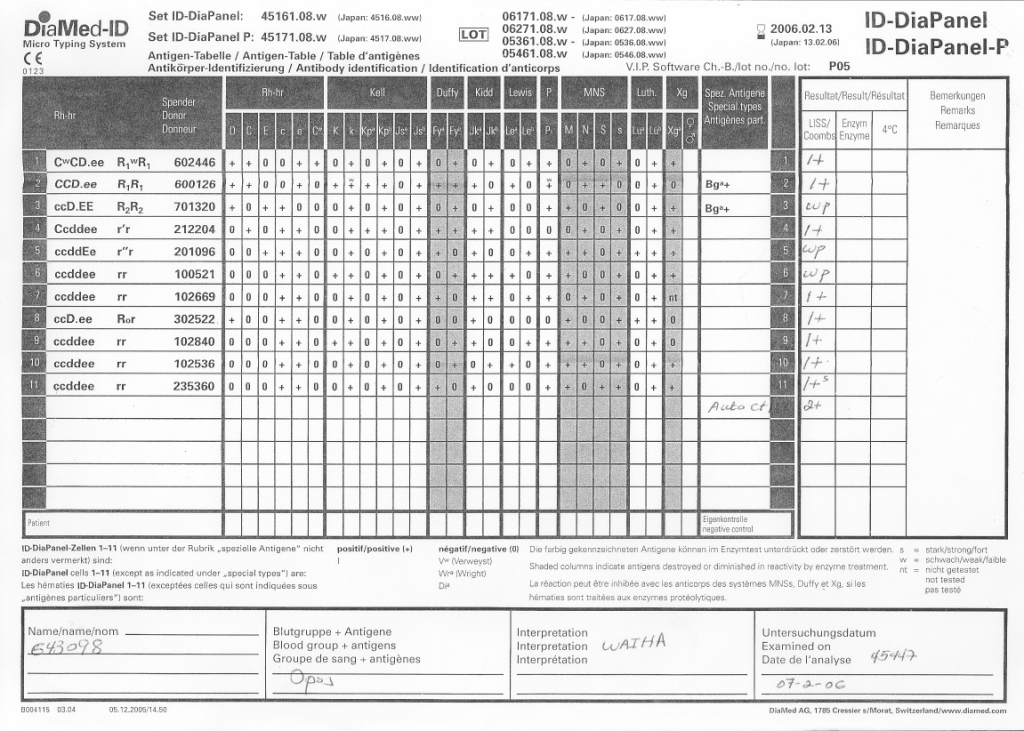
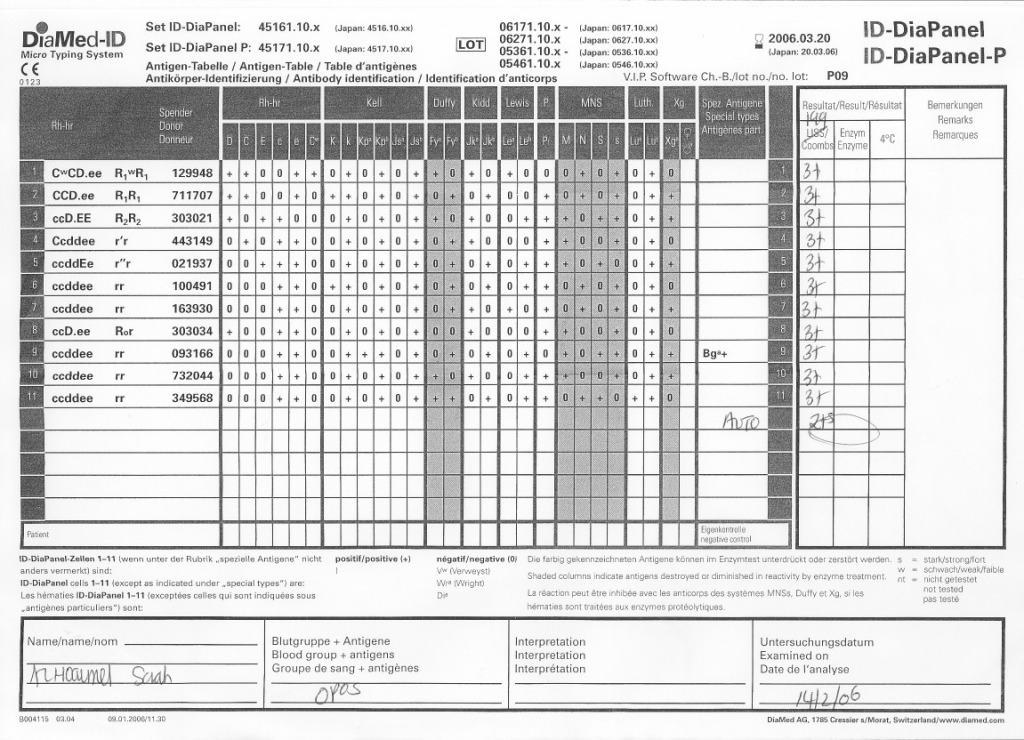
Here is the same patient with the new panel with 5 weeks before its expiration:
Donor physical examination, along with the donor questionnaire, are important both for donor and patient safety. In general:
Is it safe for the donor to donate?
Is it safe for the patient to receive the blood even if it is safe for the donor to donate.
Any donor who does not feel well must not donate. This may be the single most important step in ensuring a safe blood supply.
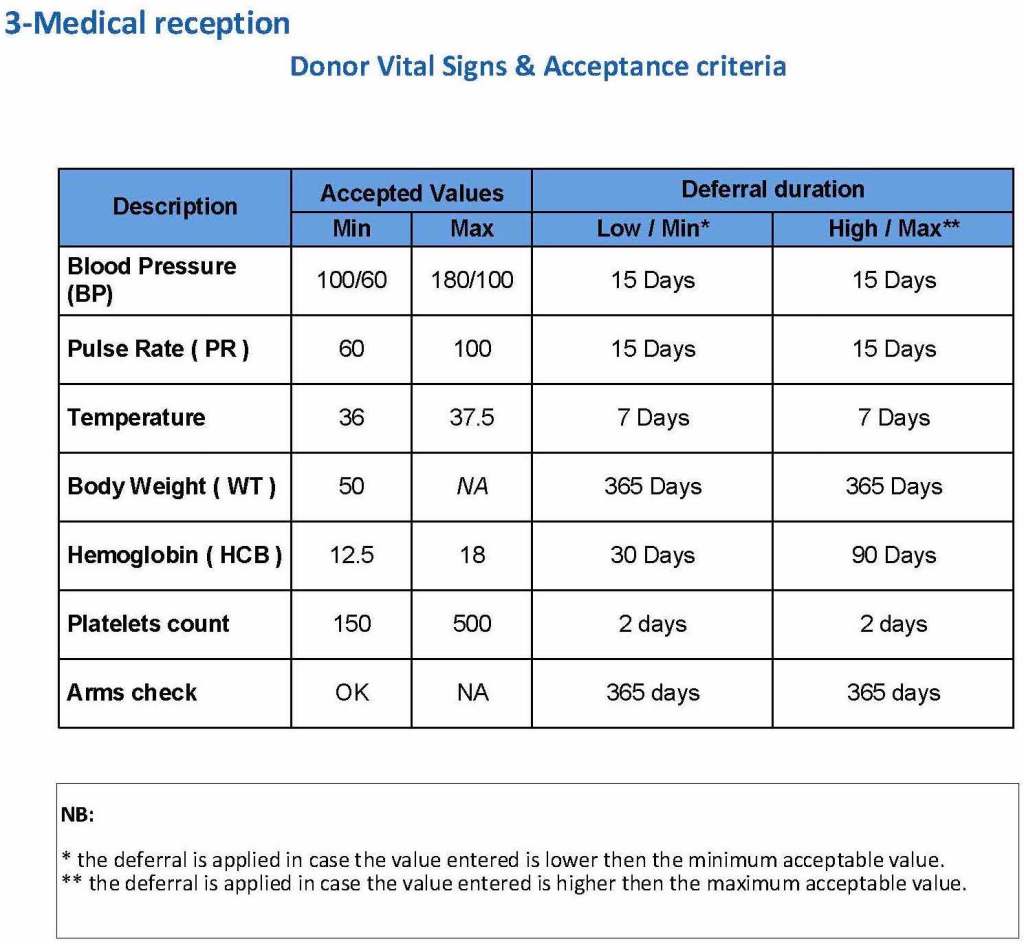
The donor physical examination includes the vital signs (blood pressure, pulse, temperature, heart rate, and temperature). I have attached a sample set of criteria for review. All are user-definable. Note how the arm examination is also included (looking for scarring, skin lesions, etc.)
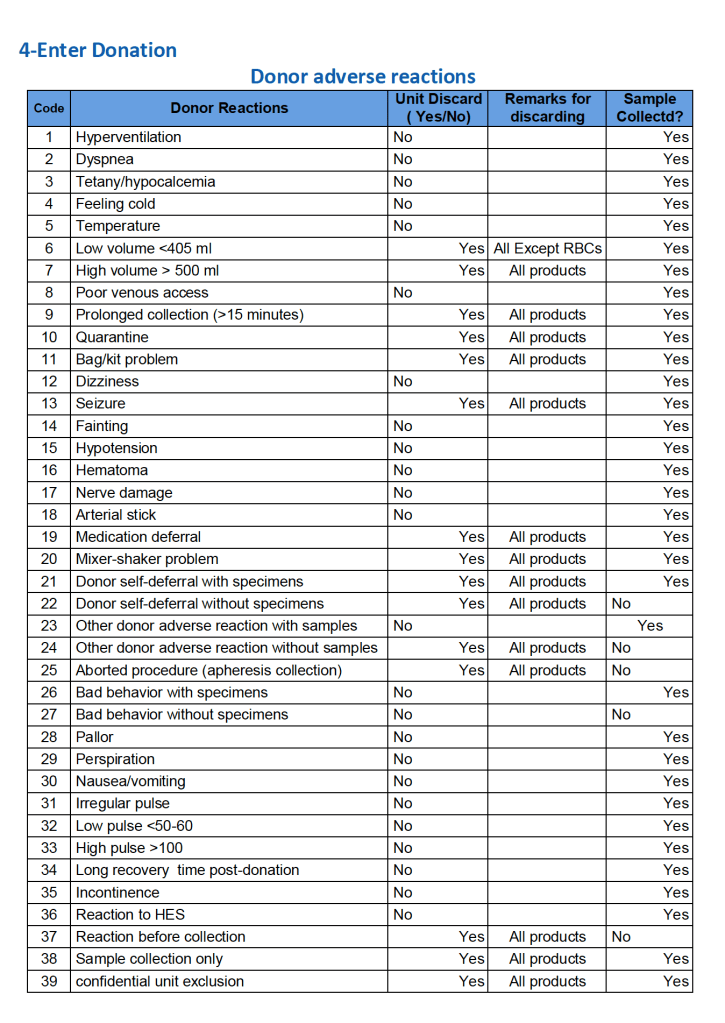
For all types of donations, there may be adverse reactions. These must be documented in the record along with the disposition of the donation. Will the donor need an extended deferral if the RBCs in the apheresis run are not returned? This can be built from the reaction documentation. Note the following sample table of reactions.
To Be Continued
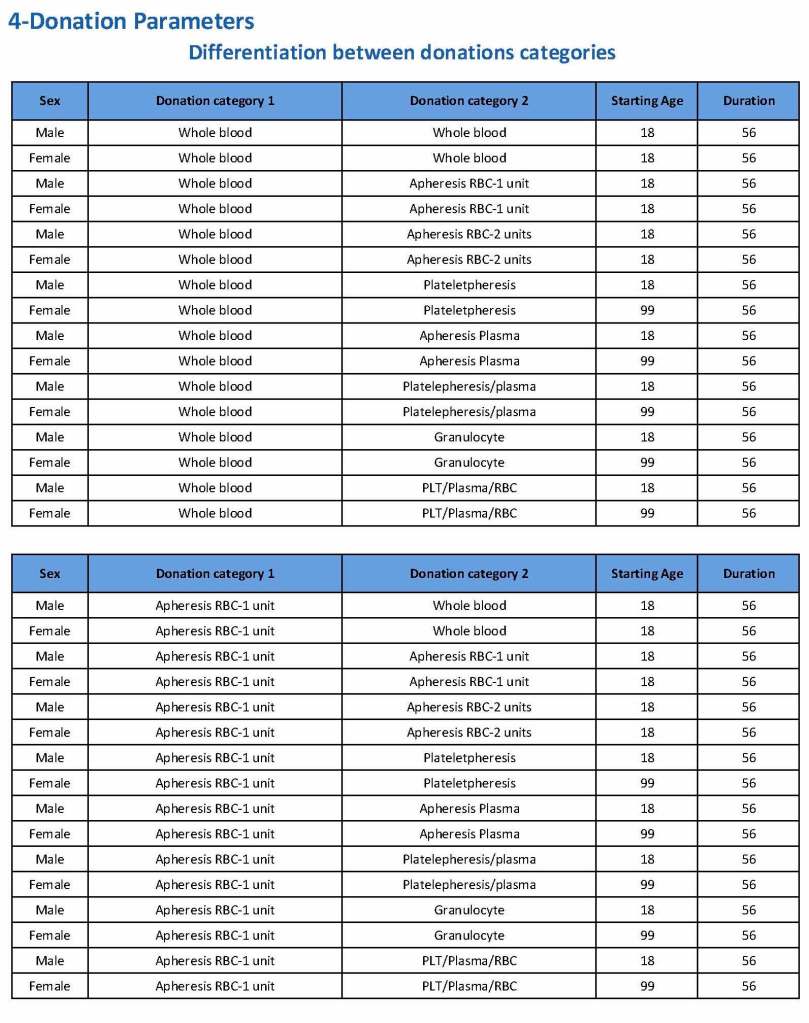
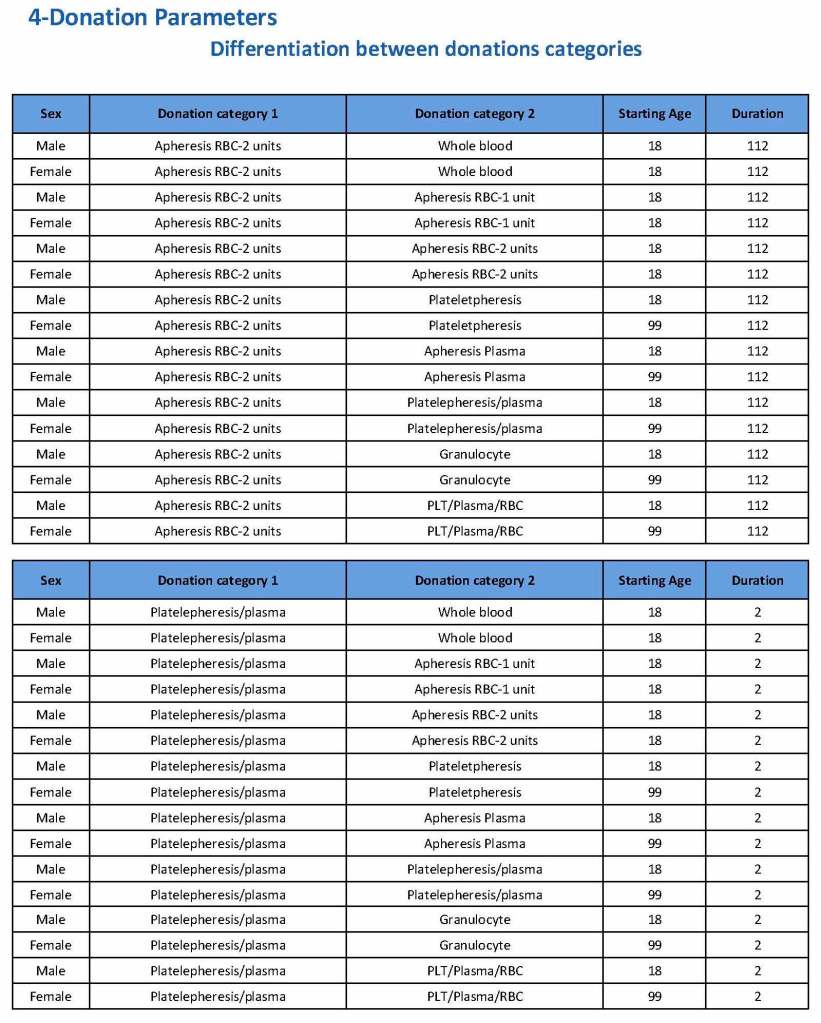
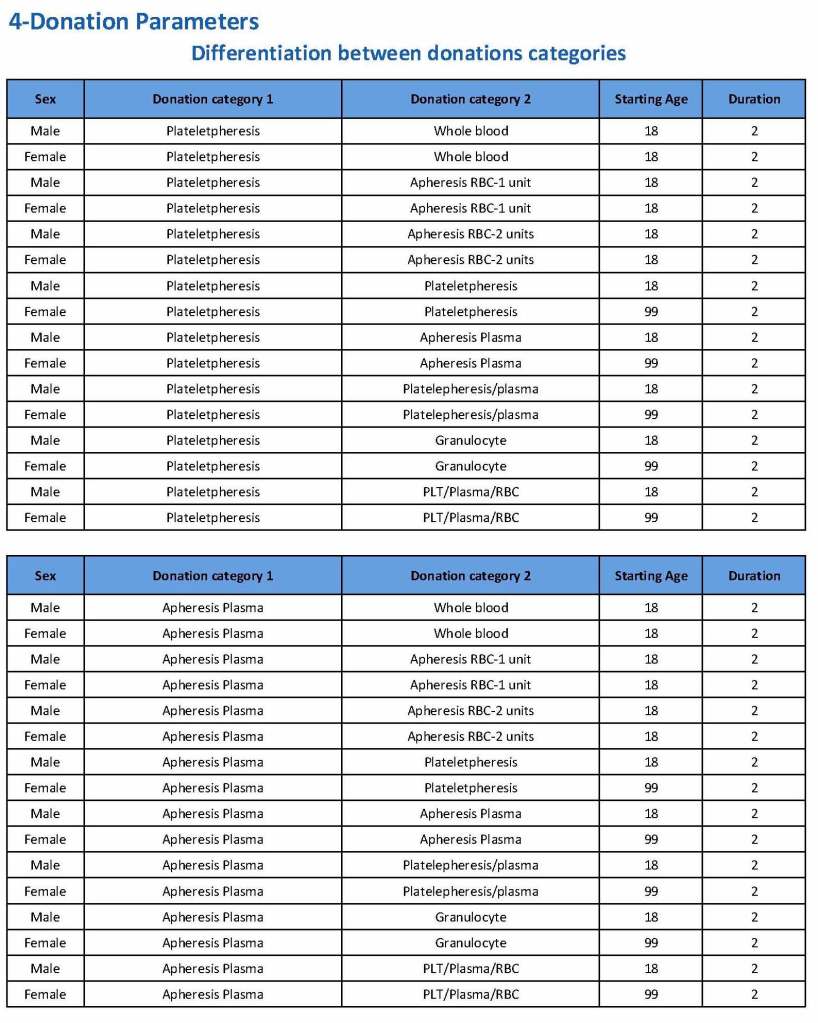
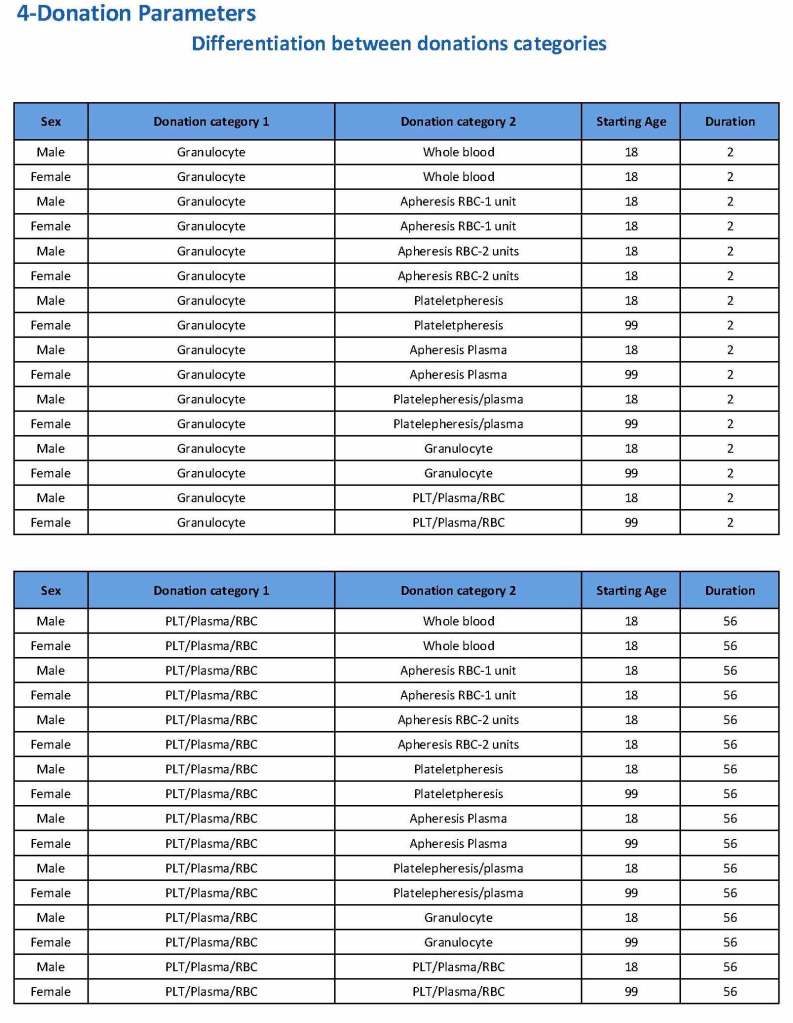
Donation can be whole blood or apheresis-based. The sex and age for each donation type is specified. At HMC, we did not accept females for platelet or plasma donations, so the starting age is listed as 99 years. Otherwise, in accordance with Qatari law, the starting age for donation is 18. All these parameters are user-definable, and a transfusion medicine physician can override the rules if necessary.
For each and every combination of donations, the deferral interval must be specified. Examples follow. The temporary deferral period is in days:
Previous donation whole blood, current donation whole blood: 56
Previous donor platelets, current donation whole blood: 2
Previous donation whole blood, current donation platelets: 56
Also note how for each possible combination there is an entry for male AND female. Females are restricted to whole blood donation and only RBCs will be made from the collection.
If there is a collection incident and the apheresis procedure is not completed, the interval will be set to 56 days. This will be covered in the post on donor adverse effect reporting.
To Be Continued: