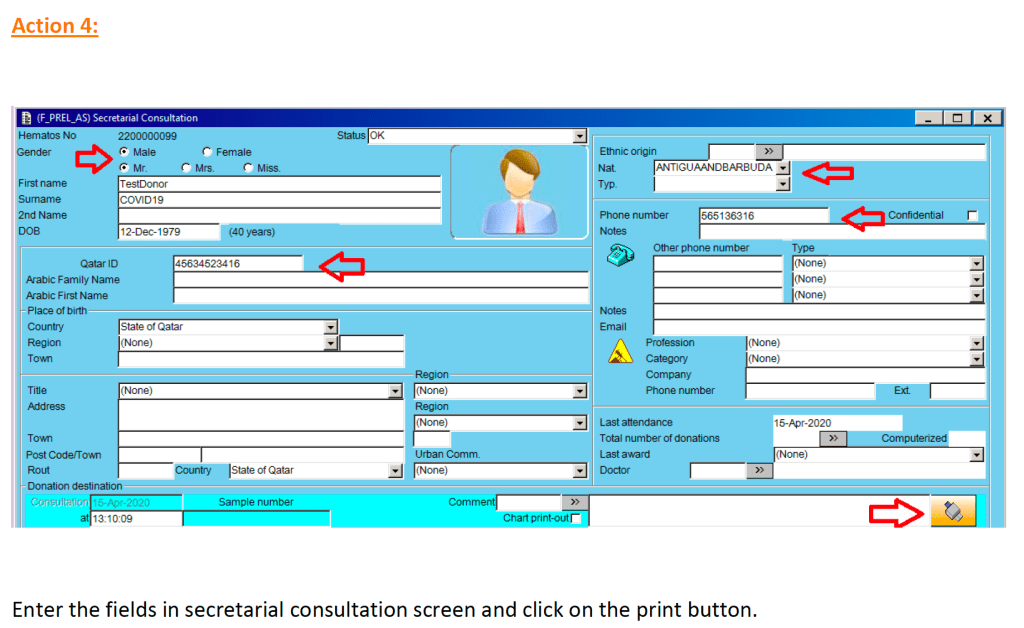
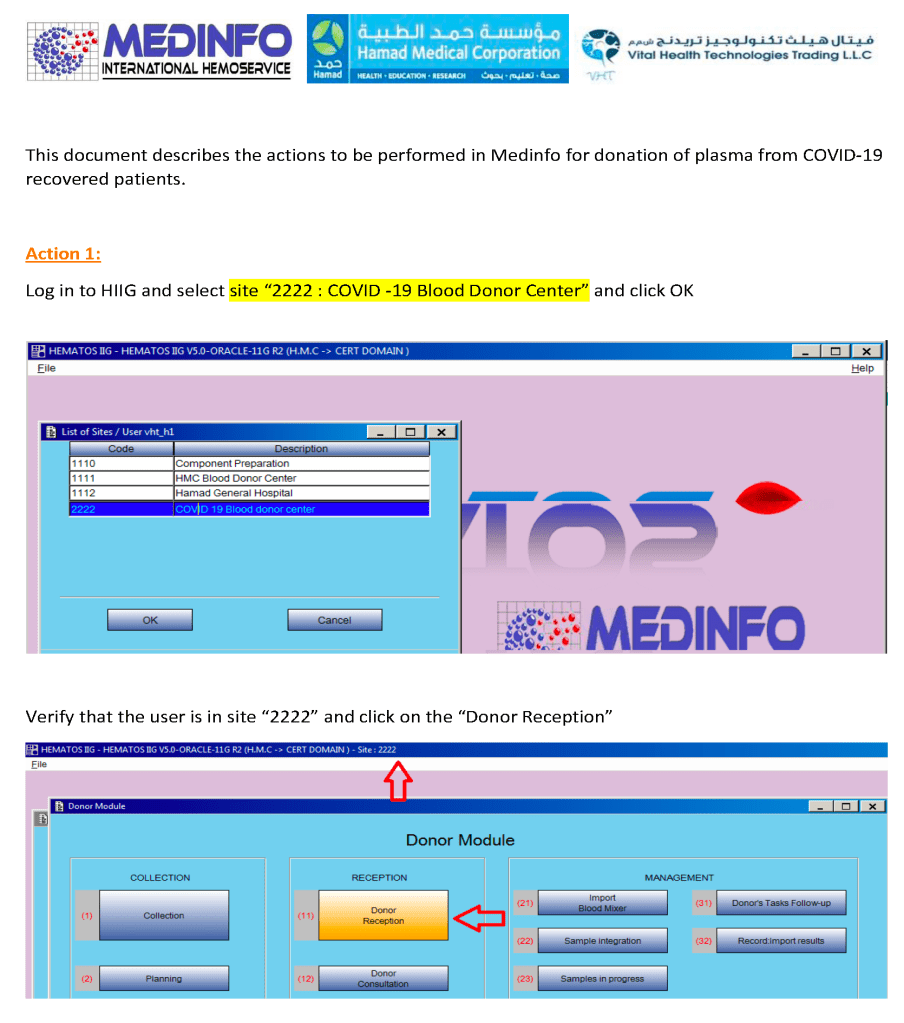
In my recent post, I provided sample flows and parameter mapping for delivery of blood components. The final components from the component preparation center may be sent to various depots (freestanding location and/or hospital blood banks. There should be complete traceability for every step (from donor reception, collection, testing, and processing) transport between locations, and finally the exact storage site, which might include which refrigerator/freezer/incubator and even shelf/position number for each component is stored. The end of that document showed rules for type/antigen matching.
For disaster planning, rapid inventory enumeration by type is very important. This can be very time-consuming manually. With our Medinfo Hematos blood bank system, we could quickly get total inventory across the Qatar or by hospital in less than one minute. We could also quickly find antigen-matched units across the system and reserve it at any one site for another if necessary.
Smart blood bank dispensing refrigerators, as offered by Haemonetics and Angelatoni, may also serve as depots and take the place of a hospital blood bank for some dispensing. These solutions can also capture vital information about the storage conditions of the components and prevent release if the storage criteria are not met. They can also interface with blood bank computer systems and use the main system’s logic for the dispensation rules. In Medinfo, they can be added as a hospital blood bank site.
Upon receipt at the hospitals from the blood processing center, the forward ABO and D typing must be confirmed. We used D reagents which detected partial D so we would call such donor units as D-positive. However, if a patient type reagent insensitive to partial D types is used, it is possible for a unit to be typed as D-negative whereas in the donor center it might be D-positive. Sometimes, nothing types consistently as D-positive: all you can say is that with a particular reagent and lot number, there is or isn’t reactivity.
The greatest complexity is for RBCs since potentially so many antigens exist. Criteria for matching/ignoring certain antigens must be made. Critically significant antibodies such as the Kell, Duffy, Kidd, and certain Rh (D and c) must be antigen matched. A robust blood bank computer system can enforce these rules.
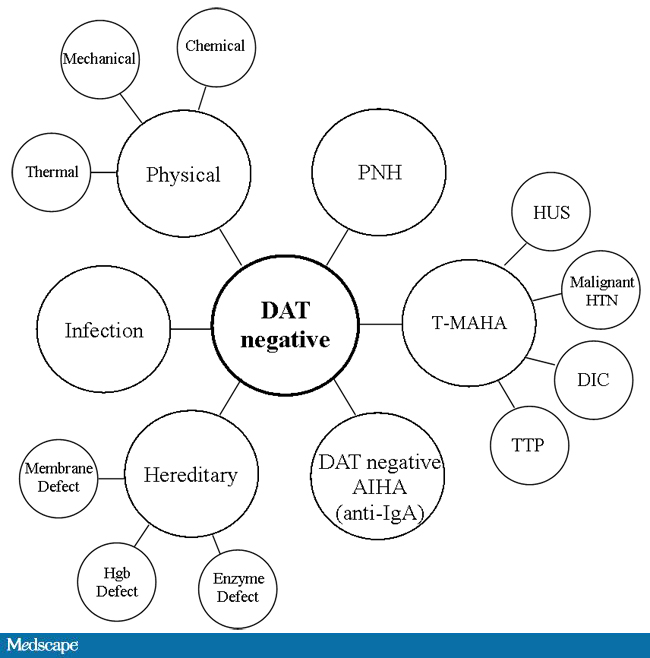
For other components, antigen/typing may be less important. In fact, in most situations, any type of platelets can be given to anyone (except neonates). Despite the potentially incompatible plasma, there is rarely significant hemolysis. In fact, if pooling platelets without regard to blood types is done, a platelet transfusion is a common cause of a positive direct antiglobulin test DAT—something that is not clinically significant. No one died of a positive DAT by itself for this reason.
Specific rules for compatible plasma types are important, but nowadays, low-titer group A plasma may be used like universal AB plasma. The challenge is to be able to perform the ABO titration (specifically anti-B) quickly—titration can be a slow process, even with automated equipment. A similar situation for low-titer, universal group O whole blood requires both anti-A and anti-B titration (I will return to this topic in a future post). With Medinfo, I can define rules (e.g. IgM titer < 1:64) to accept these units as a universal type for all ABO groups.
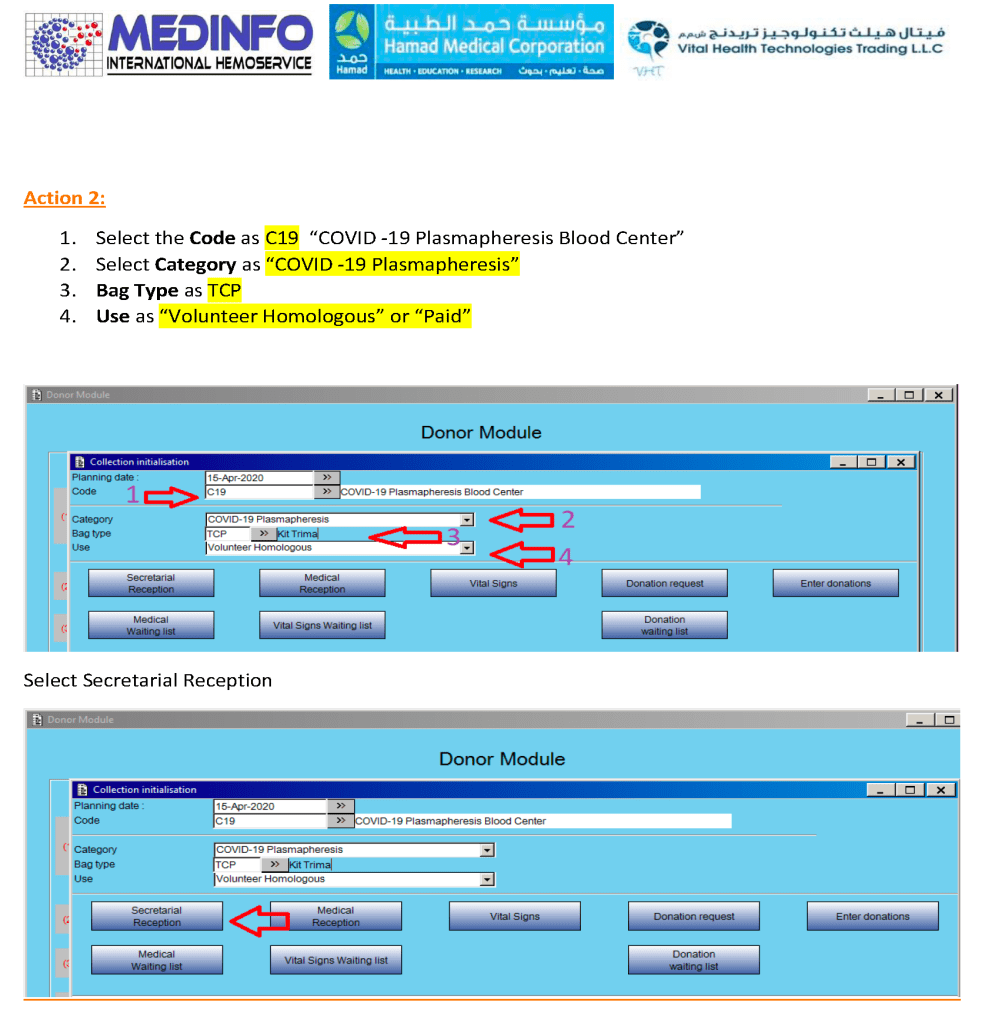
Special rules can be built into the software so that production, transfer, storage, and release of COVID convalescent plasma CCP are only performed at special quarantine sites by designated personnel. This means there can be dedicated transport pathways built into the inter-depot transfer process to keep this inventory separate at all times.