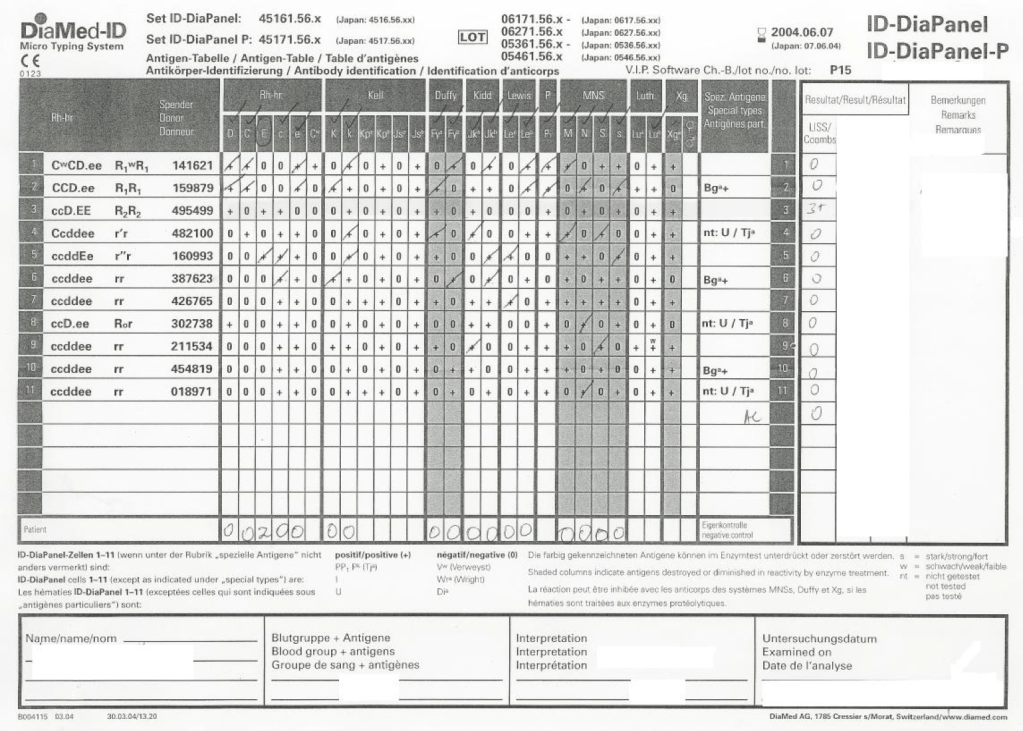
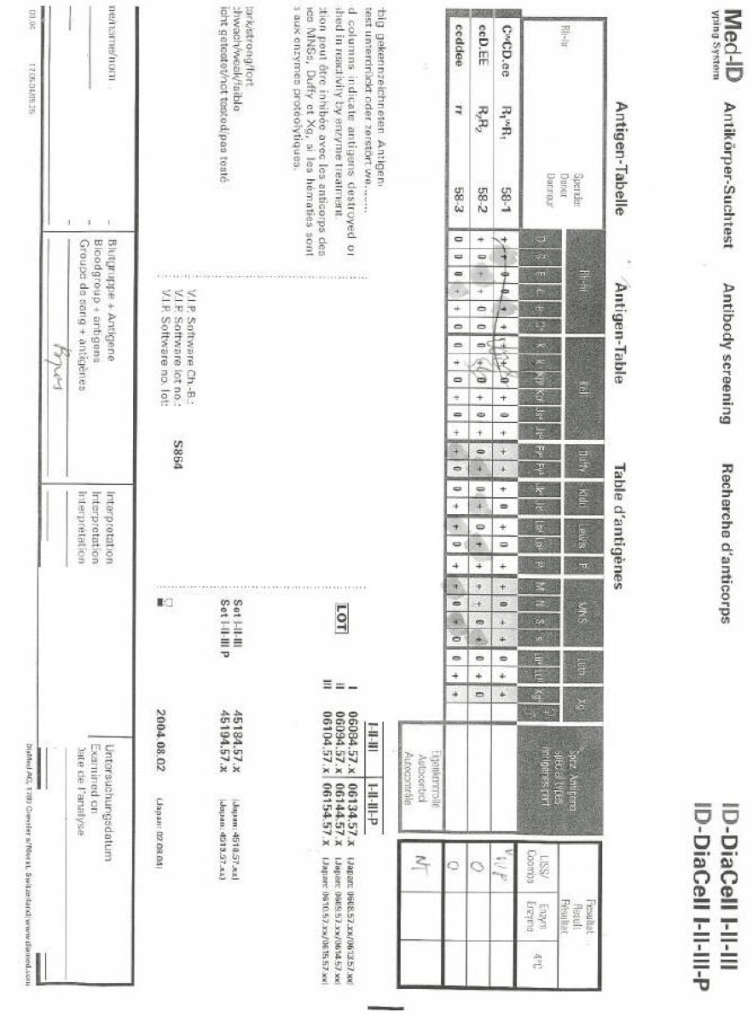
Most Transfusion Medical Directors are not information technology IT people. Still, regardless if you direct a hospital blood bank/transfusion service, a blood donor center, or both, you will still have to evaluate software for your operations. You will probably have to sign off on the selection of a system and on the final build before going live.
This can be a formidable task, especially since none of us were trained in IT. In my opinion, you can still do this based on your knowledge and experience and make a successful choice.
The most important thing is to KNOW YOUR OPERATIONS!! Someone in your organization should map out all your processes, preferably as flow charts. Optimize your manual processes. Look at your critical control points: How does the software enhance operations and safety? How does the candidate system enhance security and consistency? Does it bolster the critical control points?
My experience has been that the best software build is the one based on a good manual system. Study the new candidates: how do they enhance your operations? Now reconstruct your processes with the enhanced features of the new system.
Don’t be afraid to ask for help. Check your local resources and/or consider outside consultants if necessary. The latter should have experience in working with blood bank systems and ideally have worked with your candidate vendors.
Human beings are not consistent creatures. We often do not like following a series of steps in a processes, we like to skip around. All of this is very dangerous to patient care. The ideal system enforces consistency and integrity. I actually like it when my staff complain that the software is merciless—they cannot take shortcuts. They must follow each step in order!
Choosing a module from within a laboratory or hospital information system LIS/HIS will facilitate integration with the rest of system. However, such modules (mainly limited to hospital blood banks) do not have all the features that a dedicated blood bank software has For example, will they prevent release of unphenotyped or Kell-positive units in a patient with anti-Kell?
If you choose the dedicated system option, you must determine if a functioning interface to the LIS/HIS exists. If not, what is the time frame to make the connection? Very importantly, check that your specifications are actually built into the interface. Almost every LIS/HIS vendor says that they can make an interface, but are they communicating what you need? If you talk English and they answer you in Sanskrit, are you effectively communicating?
I prefer a dedicated blood bank system for both patients and donors, especially one that allows you to create rules to handling different situations like electronic/computer crossmatch, irradiation, etc. Integrating both patient and donor operations will facilitate operations, especially in times of disaster and product recalls/quarantines.
Make certain that there are interfaces available for your analyzers and blood production equipment (e.g. Ortho Vision Max, Reveos, Mirasol, etc.). Check these out on a site visit. Many vendors promise that they can communicate with your equipment, but you must verify this yourself.
You are the pilot. Is the transfusion or donor information organized to facilitate your decision making? Is it available all on one screen? Do you have to flip across many screens to get the information (e.g. transfusion history, transfusion reactions, DAT, antibodies, donor history, marker testing) you need?
If you are directing a donor center, you will most likely need a separate dedicated software. Usually, donor center software does not directly integrate into the LIS/HIS. However, at least your patient hospital blood bank module must be able to read your ISBT labels properly.
I recommend a visit to a site comparable to your current operations. Look for ease of use, response time, and talk privately with the end-users at the site. Ask your IT staff to help you select a site that uses the same operating and database software (e.g. Oracle) as you will be using.
How readily can the system be modified for new practices? With COVID-19, SARS, ZIKA, etc. there have been many changes in regulations in a short time. How long will it take your vendor to update your system? Is the system compliant with your local regulations and international accreditation standards?
Structurally, the optimal system is one that is a framework where almost all changes can be handled by changing settings or parameters. The underlying structure does not change so this facilitates making the modification. There is no need to “hard code” the changes. You are not writing a new software structure. Warning: many blood bank softwares do not have this framework or flexibility—it takes a long time often even to make minor changes or updates.
Using blood bank software is like playing with fire. Defects in design can adversely affect patient care. The vendor will install the software with settings, but it is still YOUR responsibility to verify it works according to the specifications.
I recommend engaging computer-literate end-users (nurses, doctors, medical technologists, recruitment staff) .from the very beginning of the actual software build. These staff can become Super Users to handle minor issues and train other staff and can help perform your software validations.
In summary, you will have to accept the choice of vendor and the final software build. Find resources to help you with these tasks. Never forget that what you are doing could adversely affect patient care if you are not vigilant!
4/11/20