As much as 90% of the RBC component allocation can be performed without an actual crossmatch test—AHG or immediate-spin provided that certain criteria are met.
Enforcing these rules, however, can be cumbersome unless one has blood bank software that verifies that each rule is met. In the Medinfo patient module, the transfusion history database is checked automatically. If the rules are met, then Medinfo allows the selection (allocation) of RBC units without performing a crossmatch test. Otherwise, it will check to see if the AHG crossmatch has been done within the past 3 days. If not, it will prompt for new crossmatch testing with a new specimen. If the situation is urgent, one can go to Emergency Mode and release components without the crossmatch.
The following is the latest document I prepared on this process before leaving HMC. Please note that there was an extensive validation before this was activated for patient use.
INTERIM POLICY: ELECTRONIC (COMPUTER) CROSSMATCH
Revised Date: 1/2/18
Principle:
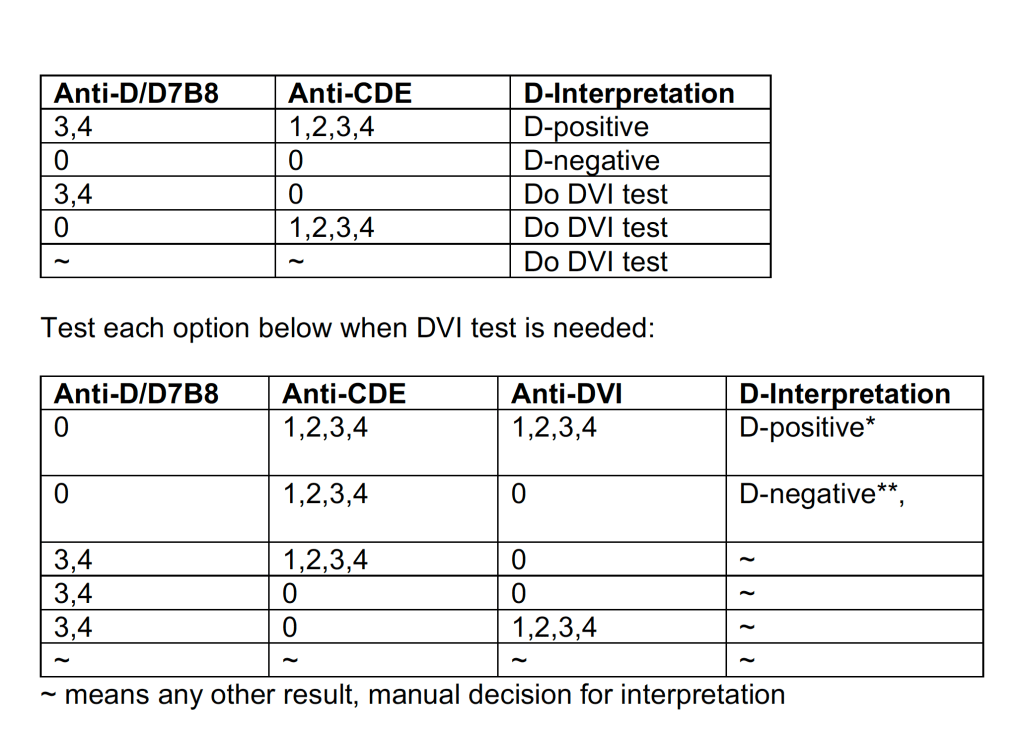
In selected patient categories, no classical crossmatch may be required for release of RBC components. The criteria are specified here as applicable in our Medinfo Hematos IIG computer system HIIG.
Policy:
- An electronic crossmatch without antiglobulin or immediate-spin phase testing may be used for the following patient categories:
- The current ABO/D type matches the historical ABO/D type.
- The ABO/D type (forward and reverse) is clearly defined without any discrepancies.
- Two determination of the ABO/D group must be made:
- One from a current specimen (within the past 72 hours)
- The second by one of the following methods:
- Testing a second current specimen
- Comparison with previous records of ABO/D typing
- Retesting the same specimen
- The current antibody screen is negative
- There is no history of RBC antibodies or a non-negative antibody screen.
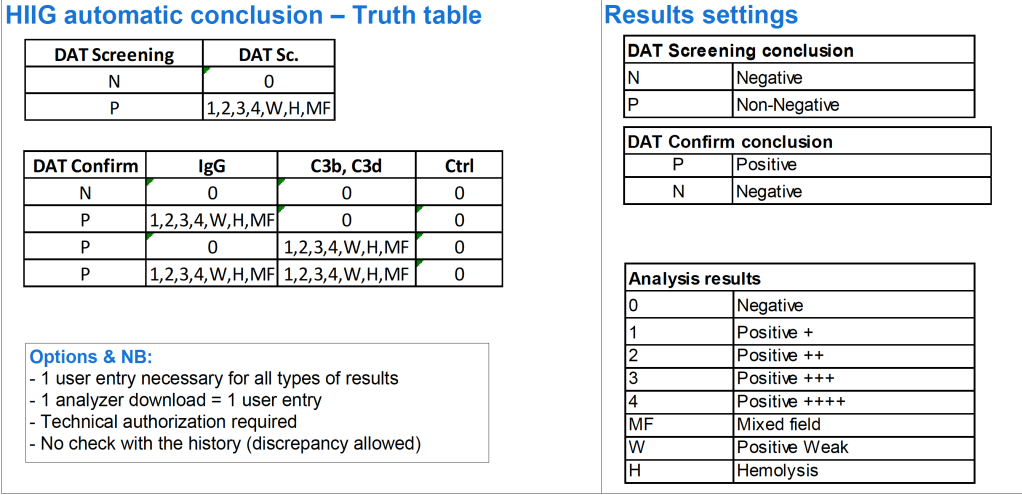
- General computer system safeguards:
- The system contains the donor unit number, component name, confirmed ABO/D typing, two unique recipient identifiers, recipient ABO/D, antibody screen typing, and interpretation of compatibility
- A method exists to verify correct entry of data before release of blood or blood components.
- The system contains logic to alert the user to discrepancies between donor ABO/D group on the unit label and those determined by blood confirmatory tests and to ABO incompatibility between the recipient and donor unit.
- HIIG will enforce the above rules.
References:
- HIIG Workflow 1004, Patient Testing, 2013
- Section 5.16, Standards for Blood Banks and Transfusion Services, Current Edition, AABB, Bethesda, MD, USA
- FDA Guidance for Industry: Computer Crossmatch (Computer Analysis of the Compatibility between the Donor’s Cell Type and the Recipient’s Serum or Plasma Type), April, 2011