Second part of fatal adverse effects due to transfusion: this was originally presented at the Saudi MOH several years ago:





To Be Continued:
12/8/20

Second part of fatal adverse effects due to transfusion: this was originally presented at the Saudi MOH several years ago:










To Be Continued:
12/8/20
The following protocol is the one I made for National Guard Health Affairs in Riyadh and has been updated to include the use of a blood bank computer system.
Medinfo has an emergency mode that facilitates release of blood components even if all the usual testing is not available
If Medinfo software is used, one can write a protocol based on the diagnosis of placenta previa to automatically allocate the blood and keep it on hold at all times.
Ward Responsibilities:
Blood Bank Responsibilities:
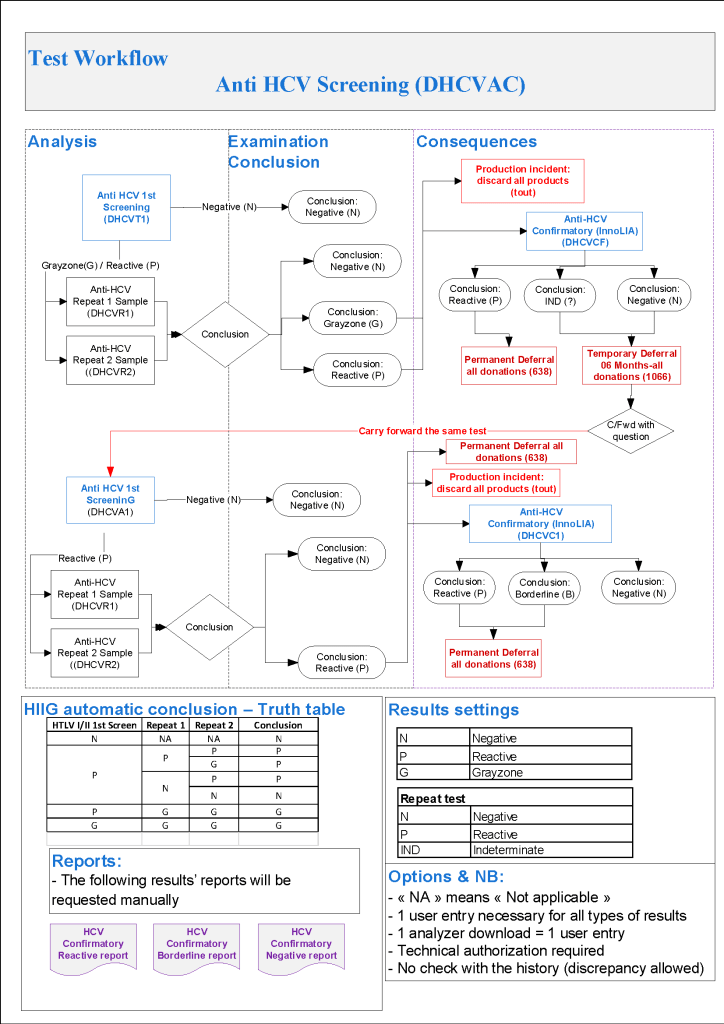
The testing algorithms may trigger additional testing, repeat of current testing at some future date, or permanent deferral. One of the most complex processes is for HCV testing. The following criteria are based on US FDA CBER guidelines, but are modified for the availability of test methodologies not licensed in the USA.
For HCV, we use the following testing in all donors:
HCV LIA is more sensitive than RIBA-3 (now no longer performed in the USA) but is not available in the USA. This test has been incorporated into the testing algorithm from CBER:
Note that indeterminate HCV results may be carried forward repeatedly by CBER rules but I decided to permanently defer the donor after 2 cycles of indeterminate results. The donor must wait SIX MONTHS before the next round of testing. Should he/she return before that time, those results may not be used for determining donor eligibility (unless the results have become clearly positive).
To Be Continued:
10/8/20
This is a presentation I made at the Saudi MOH many years ago, but it is still good for teaching purposes. I am dividing it into multiple parts because of its size.





To Be Continued:
11/8/20
Donor marker testing algorithms are very complex and serve multiple objectives:
Often the donor disposition is unclear based on a single encounter and a temporary deferral must be triggered so the current results may be compared to future ones, usually after 8 weeks, 6 months, or one year—depending on the pathogen in question.
Regretfully, the significance of reactions that do not meet the criteria for positivity may be unclear. It is very difficult to explain to the donor that he has abnormal results and cannot donate but we as physicians do not know what their significance is.
Thus, the testing algorithms may trigger current additional testing, temporary deferral with repeat of testing at some future date, or permanent deferral.
At my previous positions, I started with the AABB/FDA CBER Uniform Donor Questionnaire UDQ and then modified it to include some advanced methodologies not available in the USA.
In the next series of posts I will elaborate on the processes developed for this for each marker.
To Be Continued
9/8/20
Transfusion Medicine includes laboratory and non-laboratory functions. The non-laboratory and purely clinical functions are unique and have no analogy within the general laboratory.
The transfusion service/hospital blood bank laboratory is the closest to a laboratory operation, but there is component modification and complex manual testing, especially for reference immunohematology testing. The staff must make detailed manual decisions, the errors for which could be life-threatening for the patient.
The blood donor center manufactures a pharmaceutical, i.e. blood components with collection, donor qualification, donor abnormal results review, infectious disease marker testing, component production, and donor immunohematology testing—all subject to Good Manufacturing Practices. Never forget: Blood is a drug!!
No other laboratory section is directly responsible for treatment of critically ill patients. Therapeutic apheresis is essential for organ and stem-cell transplants, nephrology, neurology, etc. No other laboratory section is directly responsible for treatment of critically ill patients. Transfusion Medicine physicians are functioning as intensivists. There is no hiding in the laboratory from clinical medicine.
There may also be an industrial manufacturing plant to extract various blood derivatives (e.g. factor concentrates, albumin, Rh immune globulin, etc.) This is pharmaceutical manufacturing on a large-scale basis. There is medical, technical, and special administrative expertise.
Many functions may operate 24/7. The transfusion medicine physician may be on-call for donor issues and review of complex immunohematology problems to acutely decide which blood component (and phenotype) should be given as well as review all adverse reactions to transfusion.
The unique blend of clinical skills is unlike anything else in the laboratory. Also, those outside the blood bank rarely have the skills or judgments for the best course of action for transfusion medicine or for its operations.
The clinical transfusion medicine physician must make acute, life-threatening decisions unlike anyone else in the laboratory. The blood bank technologist is at the cutting edge of the battle with his testing and interpretations. No other area of the laboratory is at such risk for injuring or even killing the patient. There is high stress and burn-out.
I have talked with many blood bankers and many seem to share the exasperation that the laboratory does not understand us. The latter looks at blood bank testing like that coming off a hematology or chemistry analyzer—although patients rarely would have severe morbidity or mortality like the blood bank from errors in those analyzers.
No laboratory pathologist has the pressure of the blood bank physician on-call. It really is 24/7 and requires a broad, clinical background to make the right decisions. It is very stressful and does not permit a good night’s sleep.
Thus, I make my case to separate us from the laboratory. We can form our own more effective administrative organization and optimize our own planning. Regretfully, I have never worked in such an administrative structure. I also am a realist that cost-containment nowadays makes it much less likely high administration would permit this change for a mere cost center. This will probably never happen during my career.
Finally, Transfusion Medicine is an essential service. Blood components are essential drugs. The operations and staff must be free of political influences. This is a service for the entire region or country like the fire department, civil defense, etc.
8/8/20
Donor Collection 6
I started building this using the Uniform Donor Questionnaire UDQ from the AABB; however, I modified it to include coverage for Chikungunya, Zika, etc. and to include enhanced processes for malaria based on the Australian Red Cross.
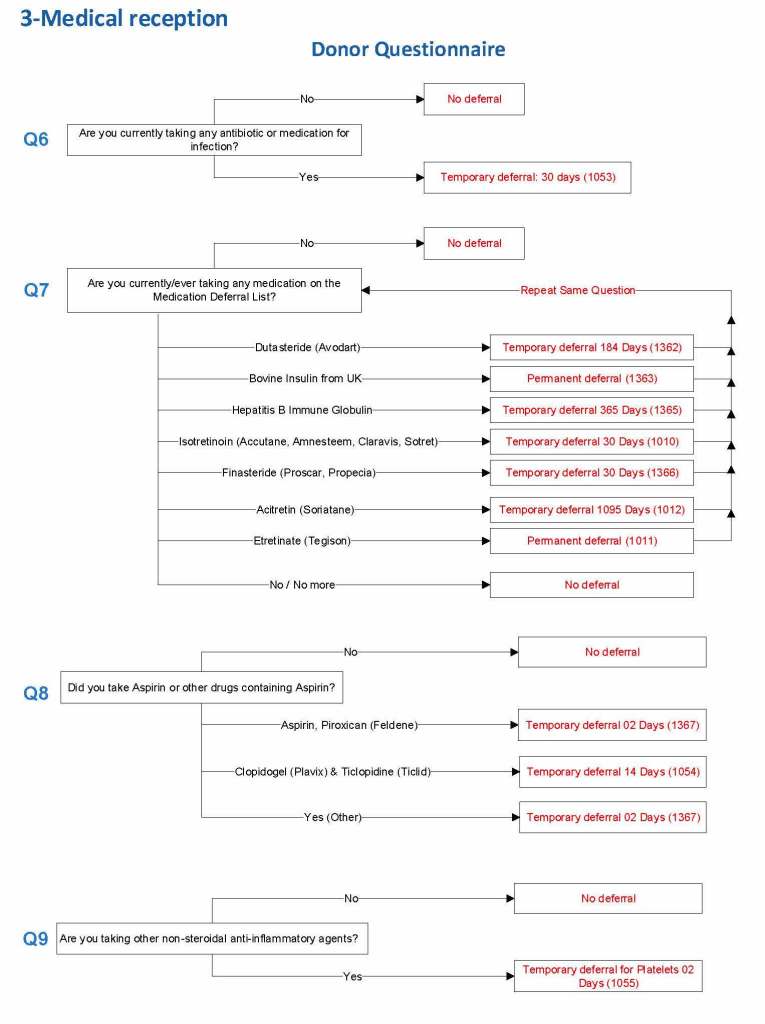
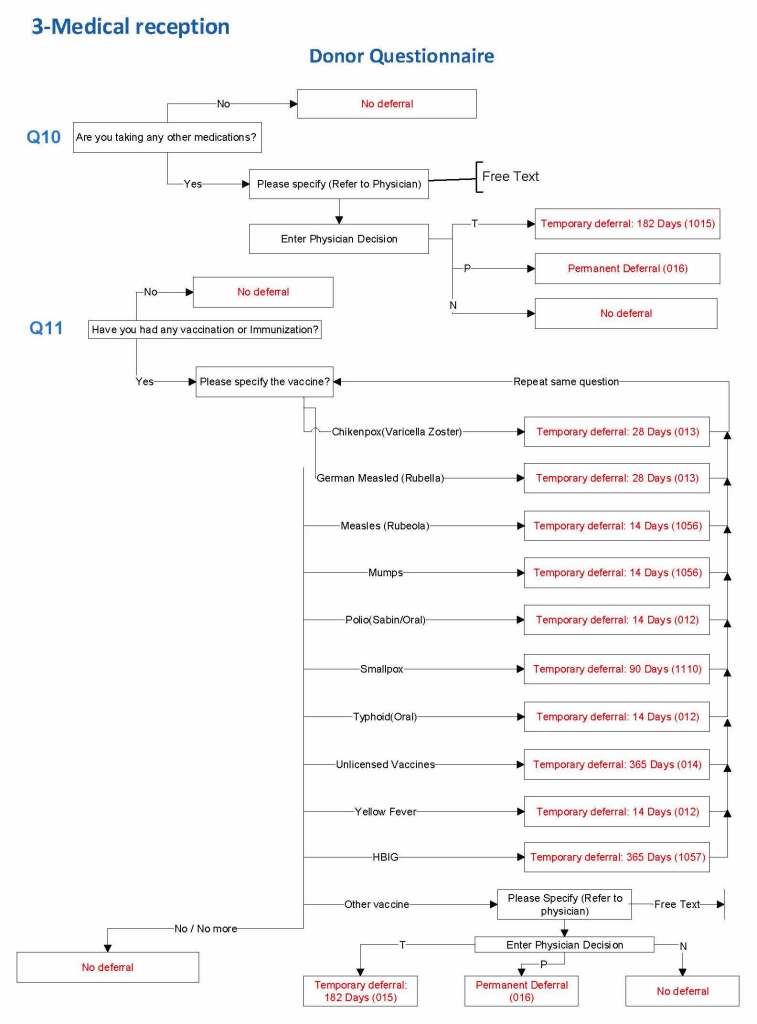
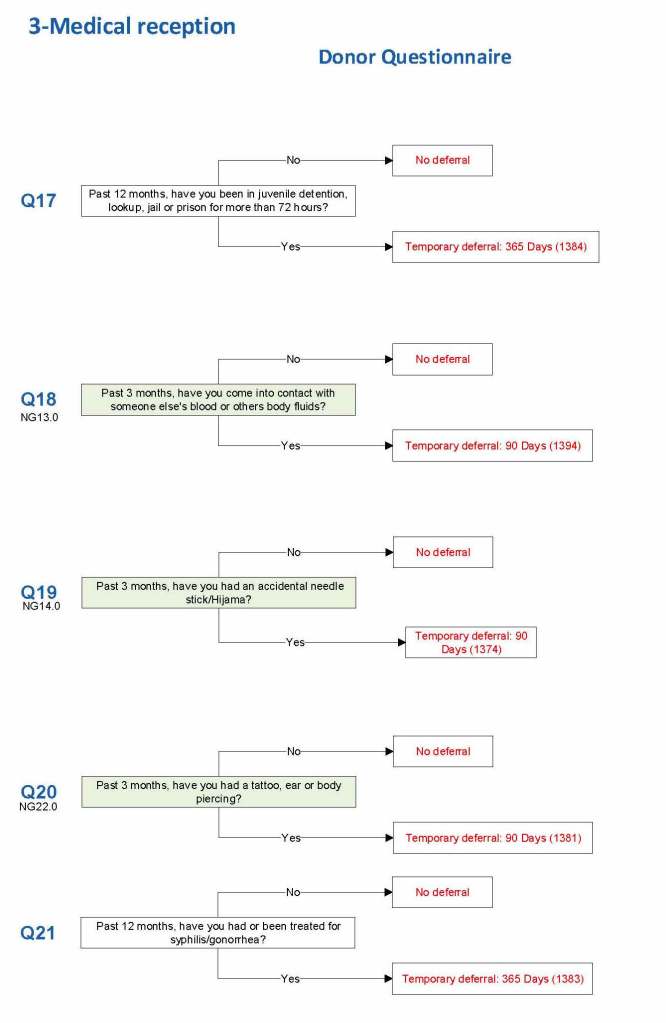
For each screening question, I prepared the exact wording (usually the UDQ’s) and set the deferral to temporary (how many days) or permanent.
Some questions were more open-ended, and the interviewer manually entered a medication, surgical procedure, etc. The transfusion physician would review this and assign a temporary (specifying the interval) or permanent deferral.
The questionnaire was constantly being updated by changes. My role was to review different accreditation systems (AABB, CE, etc.) and the World Health Organization’s websites. I would then prepare an interim policy and pass the specifications for the changes to the Medinfo software engineer and when ready, finally to the Super Users for testing. If there was an urgent change, the whole process could be completed in less than one day including validation testing.
The following shows examples of the software processes:
I emphasize that all of these settings are user-definable (at least in jurisdictions that permit all open, non-turnkey software).
Medication Questions:
Vaccinations:
Blood and Body Fluid Exposures:
Malaria Example: DMAL refers to the malaria antibody test.
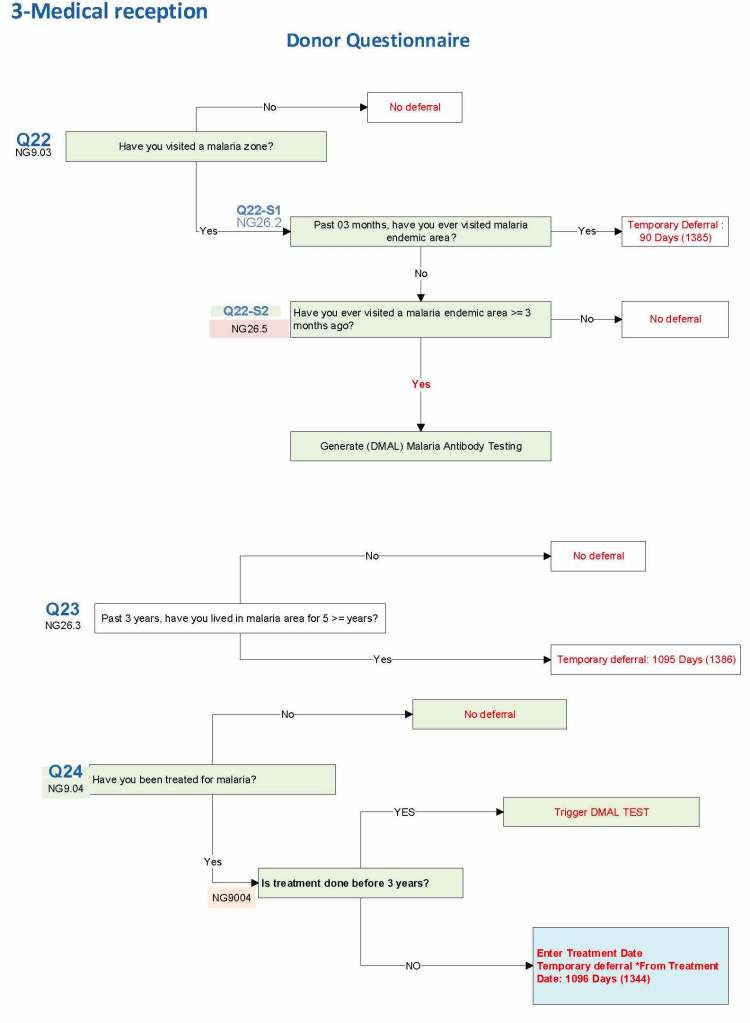
Ebola and Zika Examples:
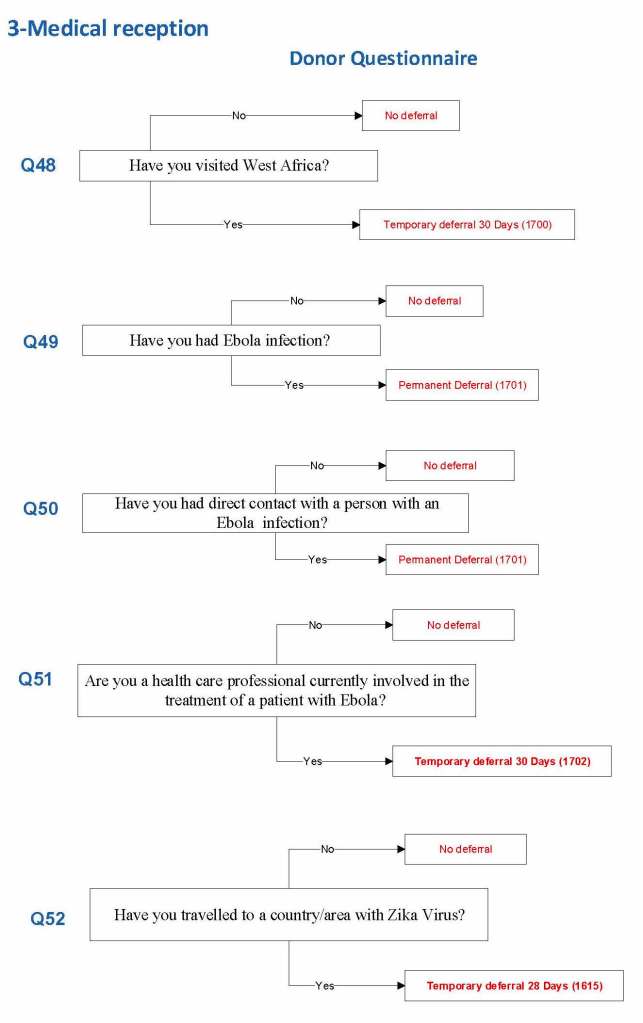
7/8/20
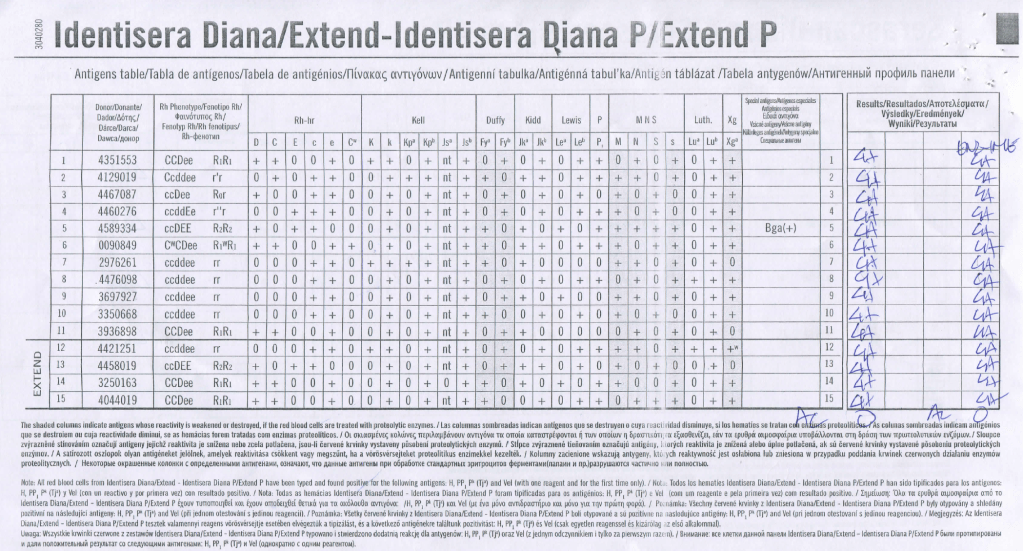
We had a mother (R1R1 K-neg) from Tamil Nadu who had several visits to our hospital. Anti-H lectin was negative. Here is a summary of her workup:
Mother’s ABO/D Typing:
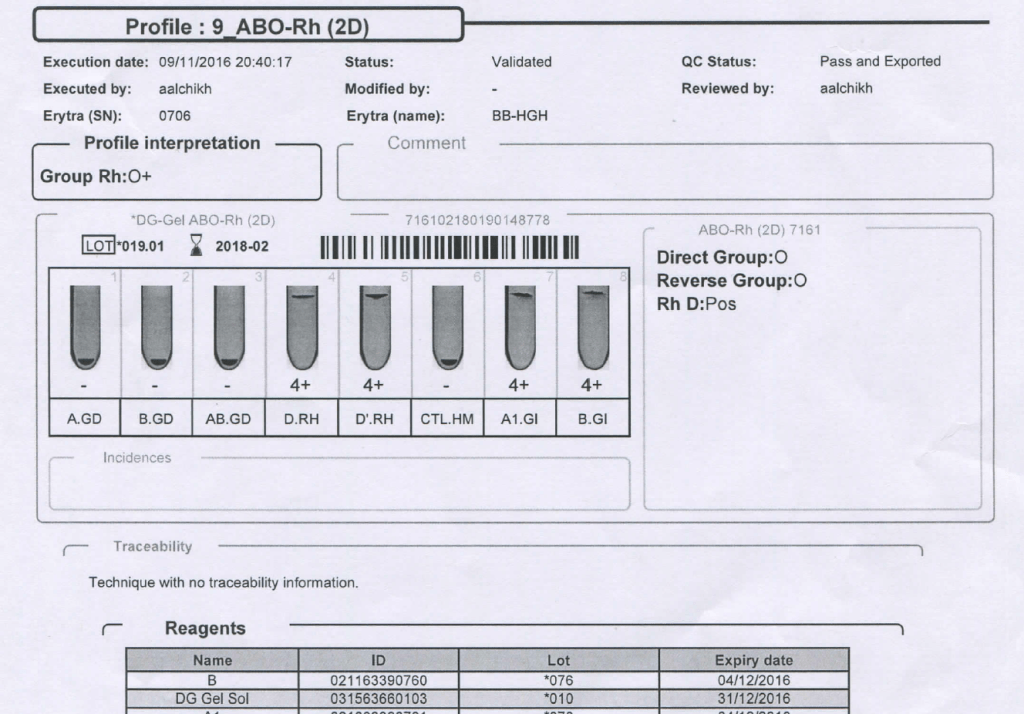
Mother’s Antibody Screen:
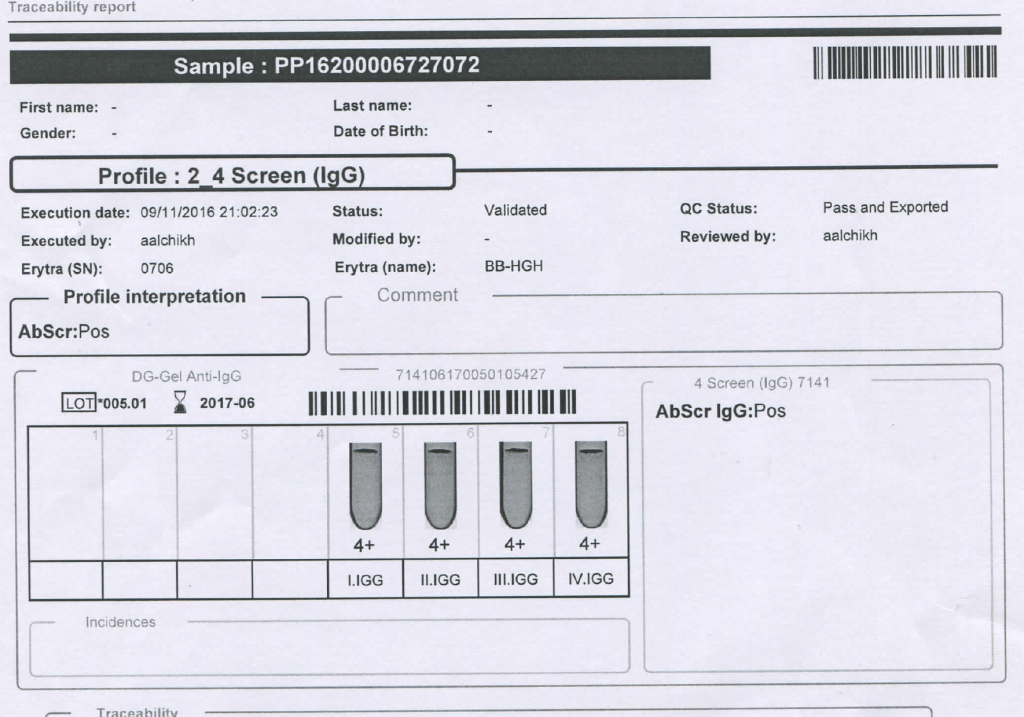
Mother’s Antibody Identification:
She gave birth to a baby girl, group B, R1R1 K-neg. This is the neonate’s workup:
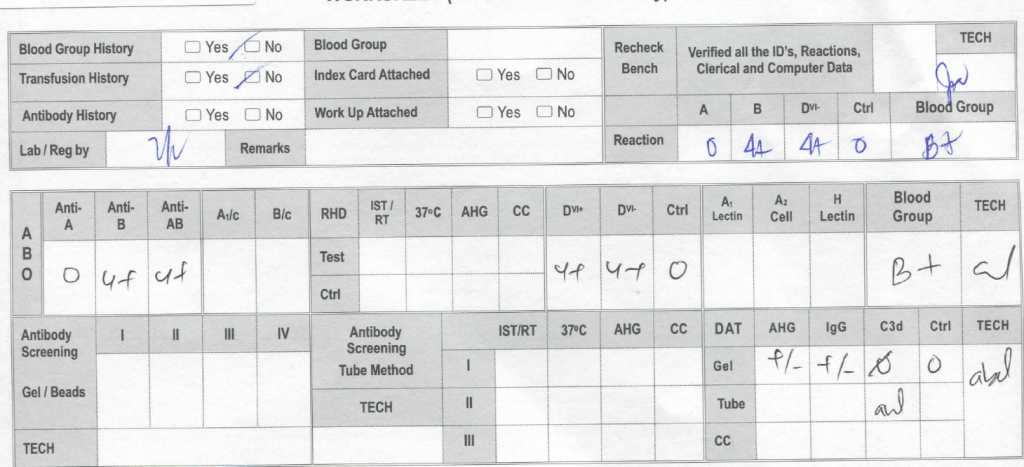
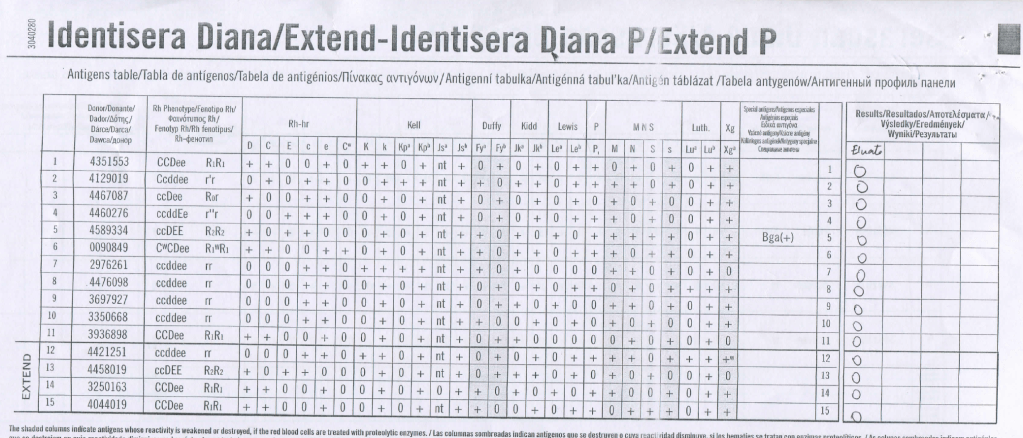
Despite the weakly positive IgG DAT, the eluate was negative. The neonate was asymptomatic.
Anti-H is mainly an IgM antibody and does not cross the placenta, thus no HDFN was noted.
6/8/20
Building the Software Processes for the Donor Collection 5: Donor Physical Examination and Adverse Reaction Reporting
Donor Physical Examination and Adverse Reactions
Donor physical examination, along with the donor questionnaire, are important both for donor and patient safety. In general:
Is it safe for the donor to donate?
Is it safe for the patient to receive the blood even if it is safe for the donor to donate.
Any donor who does not feel well must not donate. This may be the single most important step in ensuring a safe blood supply.
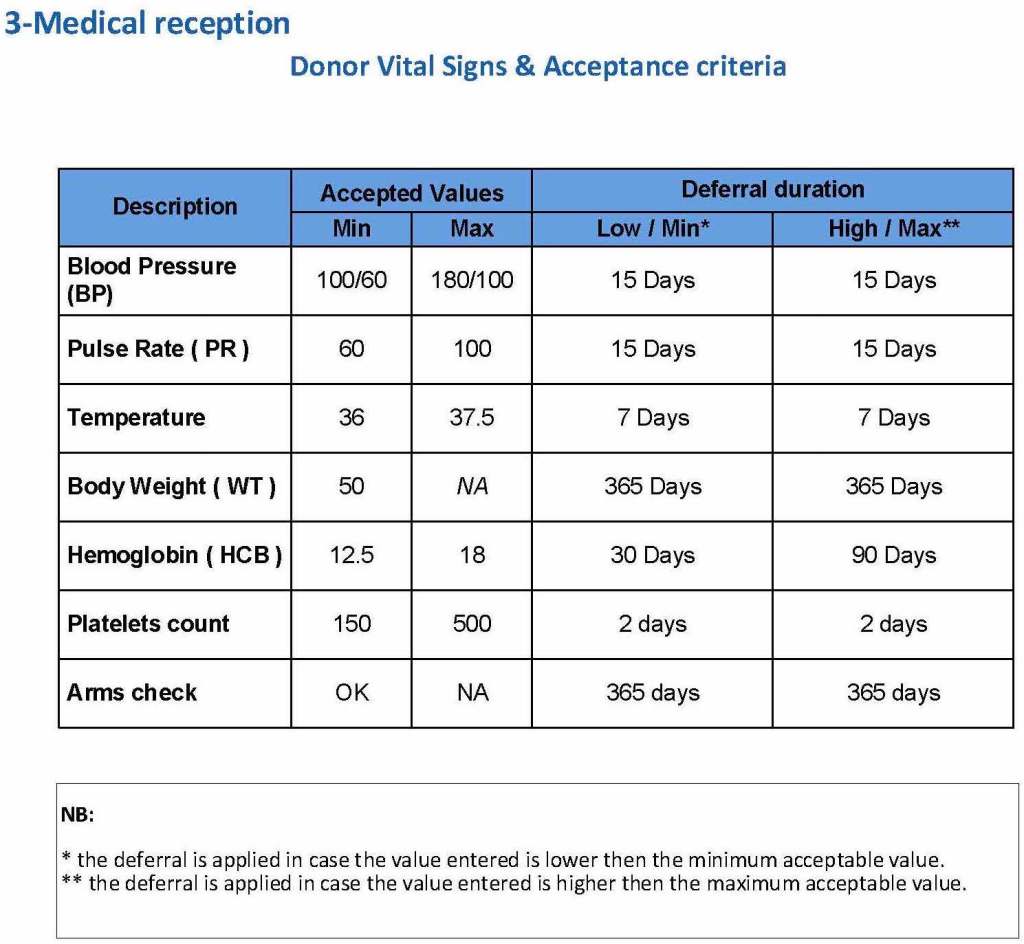
The donor physical examination includes the vital signs (blood pressure, pulse, temperature, heart rate, and temperature). I have attached a sample set of criteria for review. All are user-definable. Note how the arm examination is also included (looking for scarring, skin lesions, etc.)
For all types of donations, there may be adverse reactions. These must be documented in the record along with the disposition of the donation. Will the donor need an extended deferral if the RBCs in the apheresis run are not returned? This can be built from the reaction documentation. Note the following sample table of reactions.
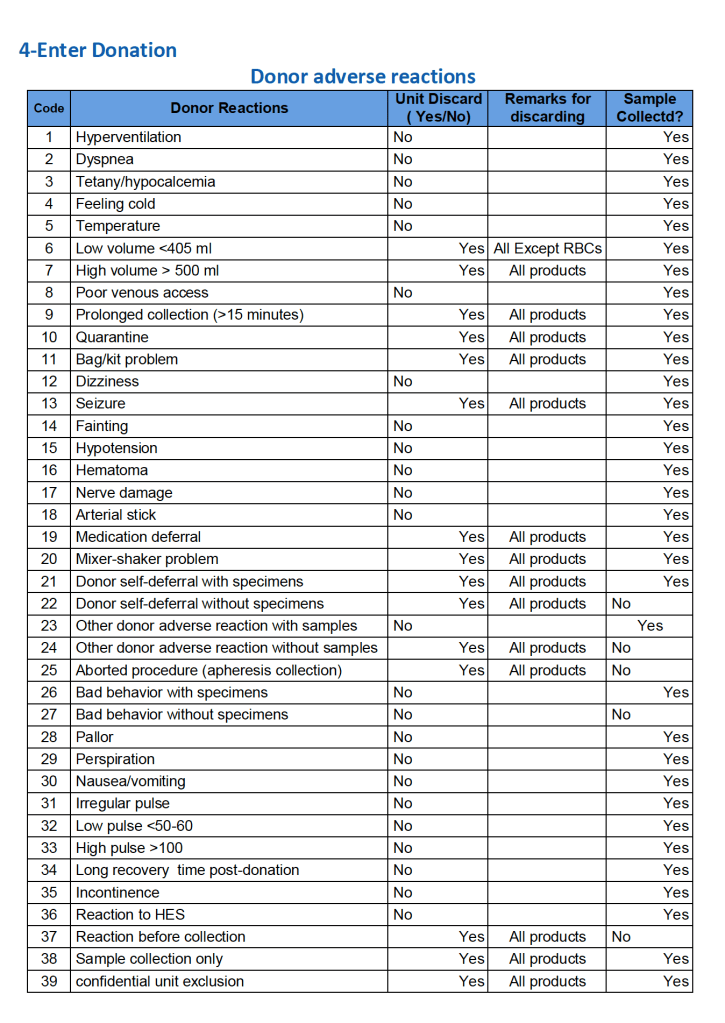
To Be Continued
5/8/20
Here is my recent interview with Terumo BCT showing how I rapidly set up the Covid-19 convalescent plasma program CCP at HMC this winter.